INTRODUCTION
Extraintestinal symptoms develop in approximately one-third of patients with chronic inflammatory bowel disease (IBD) and may involve the musculoskeletal system, skin, eyes, biliary tract, lungs, kidney, blood, or cardiovascular system. The skin is commonly affected; pyoderma gangrenosum (PG) is a skin disease that commences as erythema nodosum (nodules or pustules), followed by a rapidly developing ulcer. Between 0.4% and 2% of IBD patients are affected; the lesions are observed in 5-12% of ulcerative colitis (UC) patients but only 1-2% of patients with CrohnвҖҷs disease. The lower extremities are usually affected; rare sites include the facial and precordial areas, upper extremities, and buttocks [1]. Ulcerative PG in the breast has been associated with breast surgery or trauma, possibly accompanied by underlying immunity-mediated diseases such as IBD. However, breast PG in UC patients has been but rarely reported, mostly in females, often associated with breast surgery (although some cases were idiopathic). Most cases required additional surgical management with or without potent intravenous immunomodulators such as cyclosporine or infliximab [2-7].
We recently encountered a rare case of a male UC patient with PG of the breast, face, and lower extremities. The patient was successfully treated with steroid and mesalazine.
CASE REPORT
A 48-year-old male presented with intermittent hematochezia that had developed 3 months prior and chest wall pain that had commenced 1 month prior. He had relied on non-prescription remedies. His stool frequency was more than four times daily. He complained of painful skin lesions in the breast, face, and tibia of the left lower extremity. He had no history of gynecomastia, any other liver disease, use of drugs prescribed to treat breast disease, or prostate or testicular cancer. His testosterone and estradiol levels were normal.
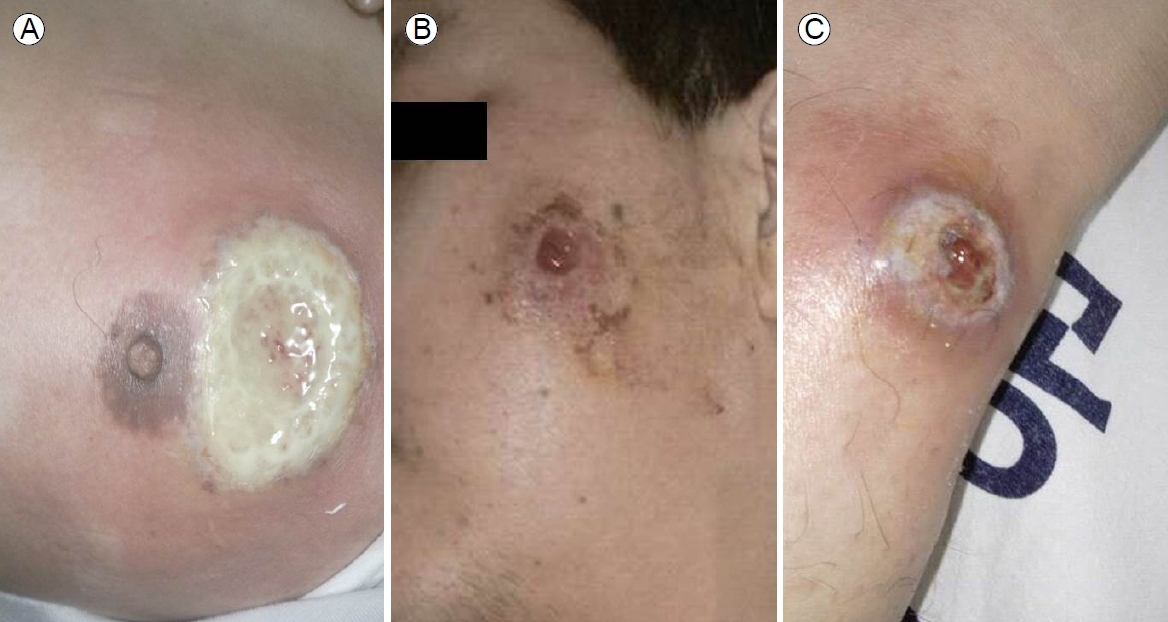
At admission, his blood pressure was 120/90 mmHg, his heart rate was 100 beats per minutes, respiratory rate was 20 per minute, and his temperature was 38.9в„ғ. Active deep ulcers accompanied by severe pain were evident in the peri-areolar area of the left breast, the left side of the face, and the tibial area of the left lower extremity. The base of the breast ulcer was covered with a thick white purulent secretion, and the surrounding area was swollen and purple (Fig. 1A). The lesions on the left of the face and anterior tibial area of the left lower extremity exhibited similar mucopurulent exudates at the bases, along with redness and swelling (Fig. 1B, C). Bowel sounds were accelerated on abdominal examination. We found no palpable mass, but the lower abdomen was moderately tender. The hemoglobin level was 11.7 g/dL, the white blood cell count was 7,400/mm3, the platelet count was 416,000/mm3, the total protein level was 5.9 g/dL, the albumin level was 3.0 g/dL the aspartate aminotransferase level was 32 IU/L, the alanine aminotransferase level was 30 IU/L, the C-reactive protein (CRP) level was 85.9 mg/L, and the erythrocyte sedimentation rate (ESR) was 80 mm/hour.
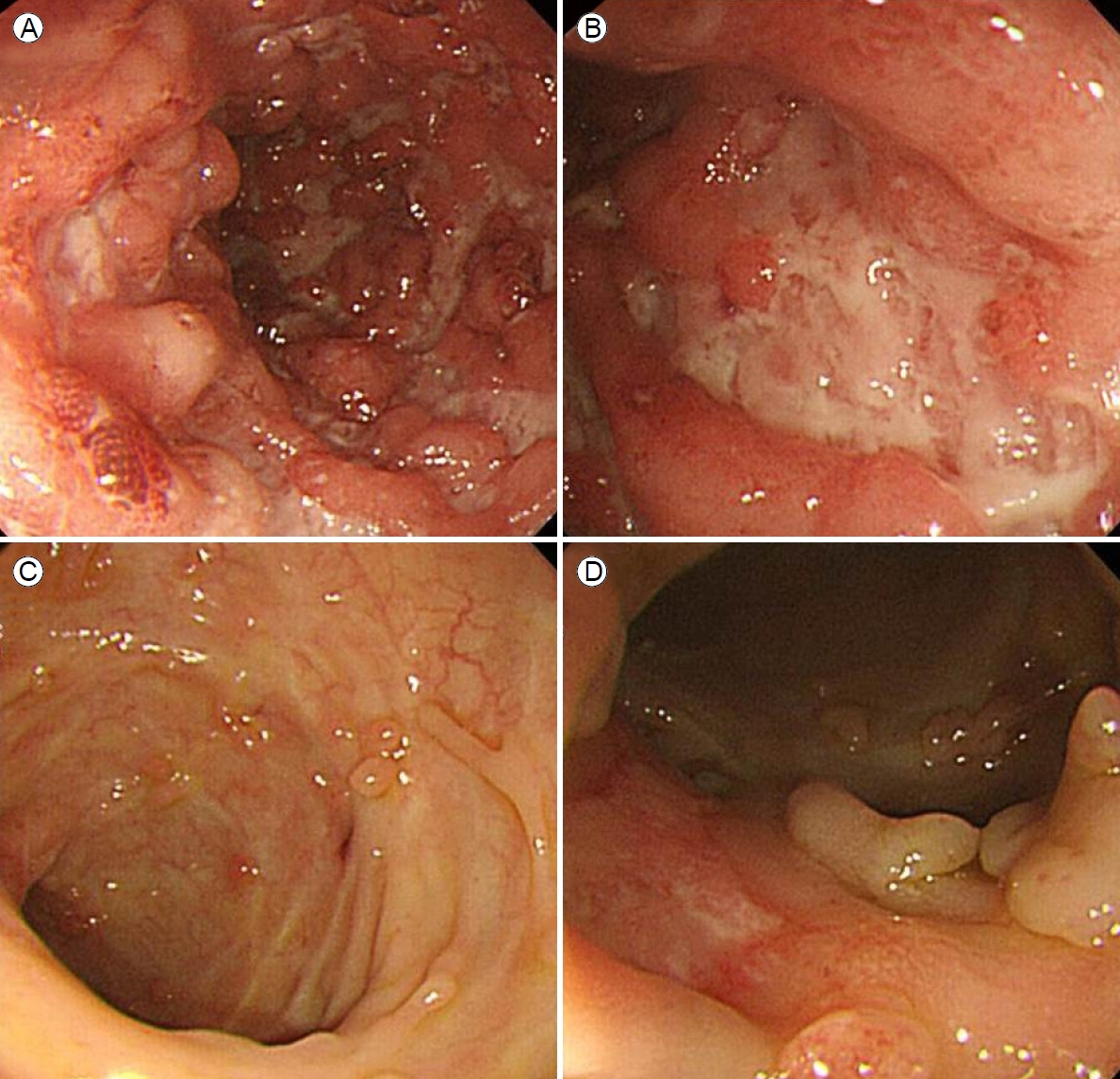
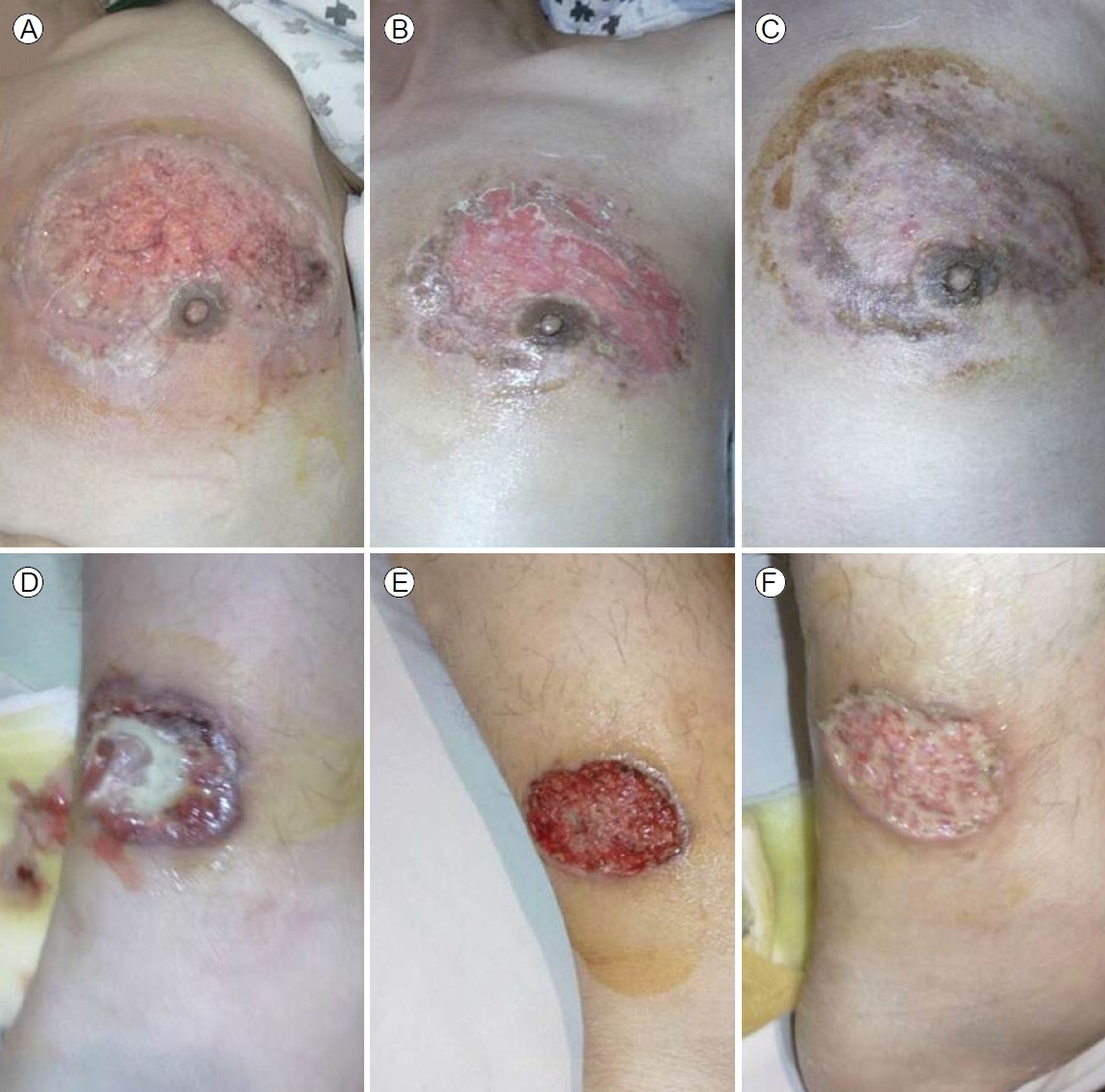
Colonoscopy revealed multiple, hemorrhagic, deep, active ulcers of various sizes and shapes accompanied by severe mucosal edema, redness, and continuous mucosal vulnerability from the descending colon to the rectum, consistent with active UC (Fig. 2A, B). Lesional biopsies revealed chronic inflammation, active ulcers, and infiltrations of inflammatory cells such as lymphocytes. The skin lesions were diagnosed as ulcerative PG accompanied by active UC. Other extraintestinal manifestations were suggestive of arthritis in both knee joints accompanied by pain during movement, but the joints appeared to be normal. We prescribed oral prednisolone 40 mg/day and oral mesalazine 2,400 mg/day and disinfected the skin lesions with antibacterial agents and a steroid ointment. Although we administered intravenous antibiotics given the possibility of secondary infection, no bacteria grew on culture; we thus stopped the antibiotics. The skin lesions began to improve 2 weeks after treatment commenced (Fig. 3).
The blood profile improved; the ESR was 35 mm/hour and the CRP level was 21.8 mg/dL after 7 days of treatment. Commencing 10 days after initial treatment, we prescribed oral azathioprine 100 mg/day to allow steroid tapering. Blood tests performed 14 days later revealed an ESR of 22 mm/hour, a CRP level of 1 mg/L, a hemoglobin level of 12.1 g/dL, and a platelet count of 419,000/mm3. The total duration of steroid use was 3 months. The bowel symptoms improved, and the patient was discharged 2 weeks later. Follow-up colonoscopy performed 3 months later and annually thereafter revealed improvement of the UC, with multiple pseudopolyps evident over the entire colon, predominantly on the left side (Fig. 2C, D). The patient was under outpatient follow-up for 28 months without any deterioration of the skin lesions. He remained on maintenance oral azathioprine (50 mg/day) and oral mesalazine (1,600 mg/day).
DISCUSSION
IBD is often accompanied by extra-intestinal symptoms in addition to intestinal symptoms including diarrhea, bloody excrement, and abdominal pain. Extra-intestinal symptoms develop in approximately one-third of cases; the skin is frequently affected. The most serious skin lesion is PG, which commonly develops after erythema nodosum in IBD patients. PG was first described by Brunsting in 1930 as a necrotic ulcer with a mucopurulent exudate, usually located in the lower extremities, rarely in the facial area, chest, upper extremities, or buttocks [8].
Several case reports of breast PG in UC patients have reported. Some developed after breast surgery when bowel symptoms were lacking, but three cases were detected incidentally in the absence of prior breast injury or trauma. Most cases were females, and the reason for this remains unclear. However, this may reflect prior breast surgery and the unique histology of female breast tissue (compared with male tissue). Notably, invasive surgery was required by all three cases, two of which also required anti-tumor necrosis factor therapy (infliximab) or cyclosporine A [2-7]. In contrast, our case responded well to steroid and azathioprine. Patients resistant to or dependent on steroids are often treated with an immunomodulatory drug such as azathioprine, which is also prescribed for patients experiencing frequent UC relapse [9]. Azathioprine has been used to treat PG, but it acts more slowly than steroid [10]. The drug is optimally used as an adjunctive or steroid-sparing agent, rather than monotherapy. Of course, the therapeutic effect is sometimes unpredictable as not all patients respond to the therapy.
Usually, PG commences as a solitary or as multiple erythematous papule(s)/pustule(s); epidermal necrosis follows within a few days, with deep violaceous ulceration. The base of the ulcer contains mucopurulent material and is very painful. The lesions look вҖңdirtyвҖқ, but most are sterile. The lesion may be pustular, vesicular, or proliferative, rather than ulcerative (the latter is typical). Our case exhibited typical ulcerative breast PG [1].
Exclusion of other diseases is required for definitive PG diagnosis. The differential diagnosis should include vascular occlusive disease, vasculitis, a tumor, infection, and tissue damage of external origin. A detailed medical history, biopsy, and bacterial culture may be required. Local ulcer treatment is crucial and gentle care essential; a steroid may be applied to or injected into the lesion. To reduce steroid use, immunomodulatory drugs such as azathioprine, cyclophosphamide, mycophenolate mofetil, and methotrexate can be considered [8,9].
In summary, PG presenting as an extra-intestinal manifestation of UC is a devastating, ulcerative, painful skin lesion that usually spreads rapidly after commencing as nodules and pustules. The lower extremities are usually affected; rare sites include the upper extremities, facial area, ears, precordial area, back, and buttocks. We report a rare case of a male patient with active UC who developed PG of the chest wall (near the left breast) and a lower extremity. The lesion was successfully treated with oral steroid, mesalazine, and azathioprine; no surgery or potent drug was required.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print






