INTRODUCTION
Primary pancreatic lymphoma (PPL) is a rare form of extranodal malignant lymphoma, comprising 1% of all extranodal lymphomas and 0.5% of all pancreatic masses [1,2]. Because PPL and pancreatic adenocarcinoma have similar clinical presentations and imaging findings, histological examinations are needed to differentiate these cancers [2-4]. Differentiation of PPL and adenocarcinoma is important because of the different treatment options that improve prognosis for affected patients [3]. We report a rare presentation of lymphoma as a primary pancreatic tumor involving the stomach.
CASE REPORT
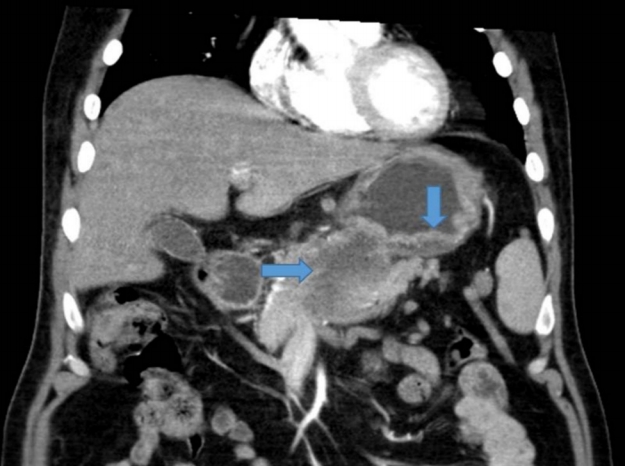
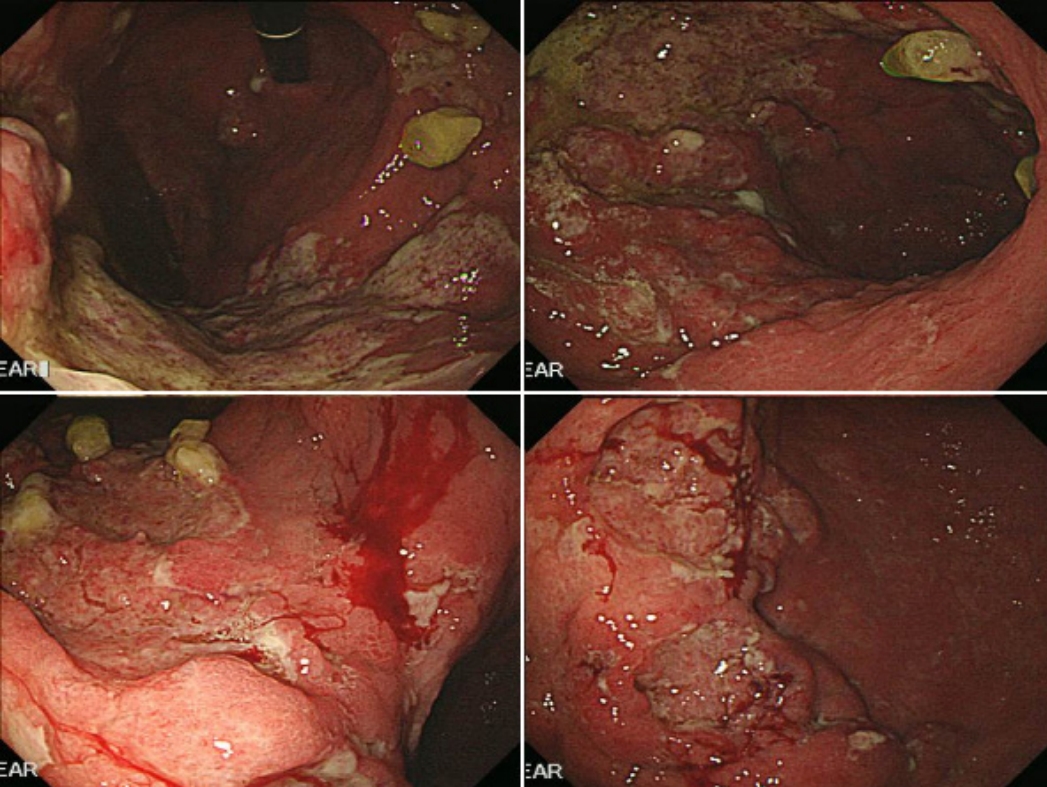
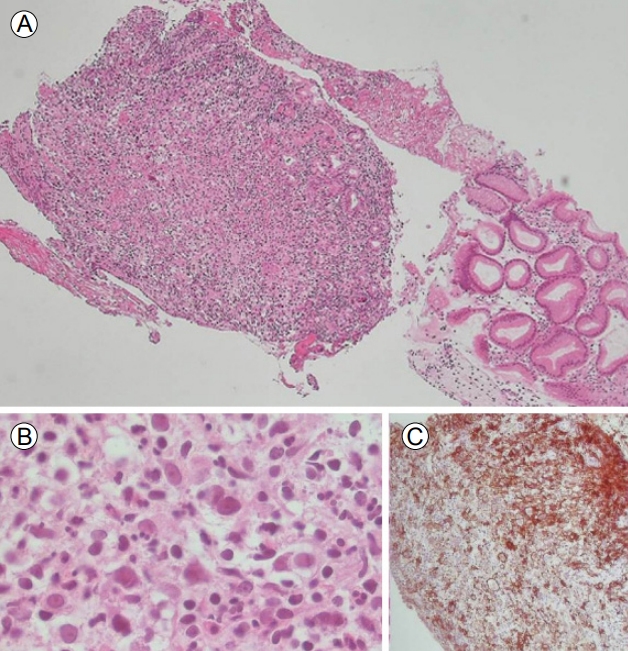
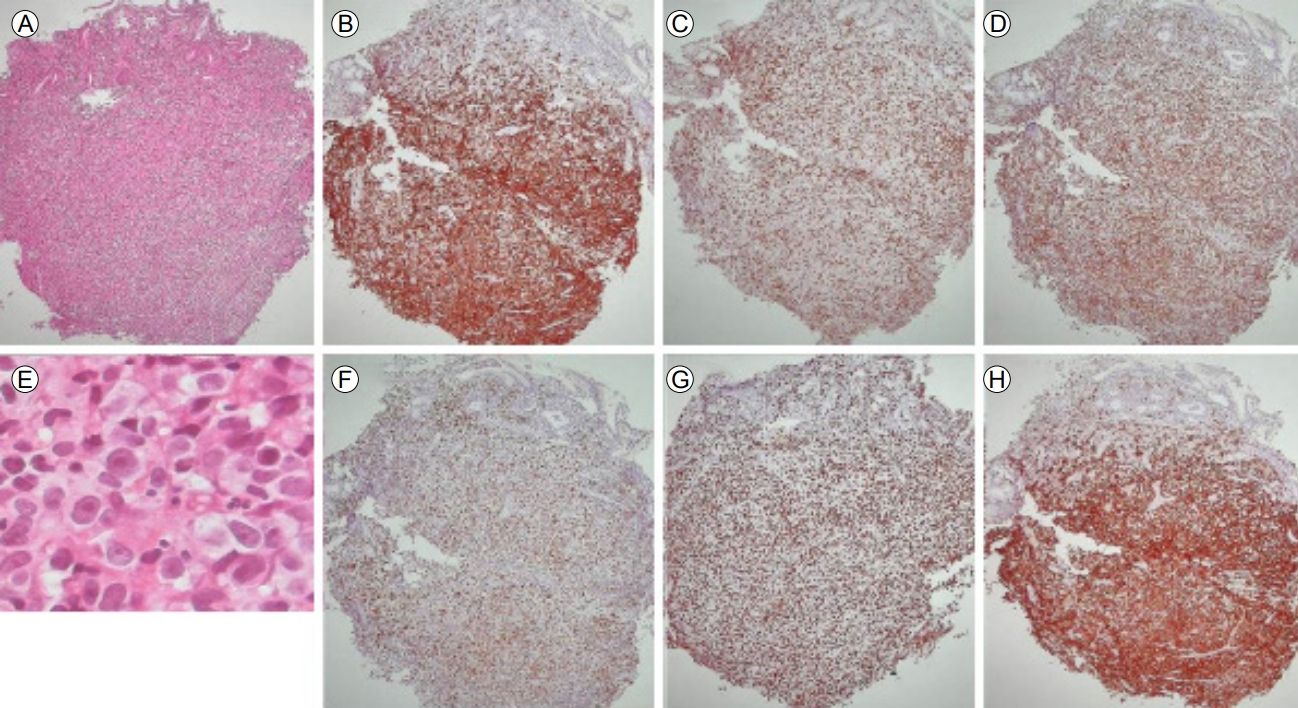
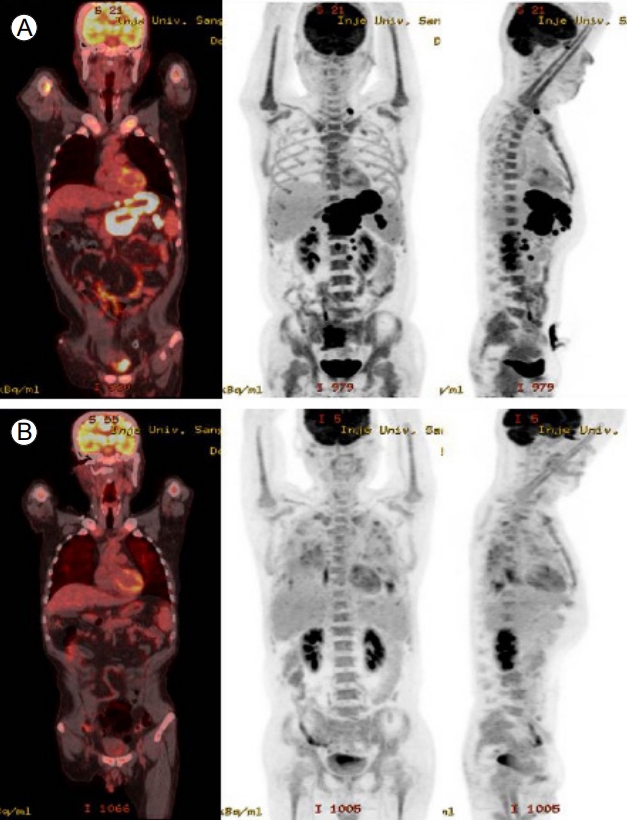
A 46-year-old man presented with indigestion, epigastric pain, and weight loss for 2 months. He had a history of schizophrenia, for which he was taking a medication. On physical examination, he exhibited tenderness in the epigastric area, without rigidity or rebound tenderness. The results of laboratory tests conducted at admission were as follows: hemoglobin, 12.2 g/dL (normal range, 13–17 g/dL); white blood cells 9,260/µL (4,000–10,000/µL); platelets, 319,000/µL (150,000–400,000/µL); aspartate aminotransaminase, 20 IU (0–40 IU); alanine aminophosphatase, 15 IU (0–40 IU); lactate dehydrogenase, 398 U/L (180–430 U/L); gamma-glutamyltranspeptidase, 74 U (0–60 U); total bilirubin, 0.4 mg/dL (0.2–1.2 mg/dL); direct bilirubin, 0.1 mg/dL (0–0.2 mg/dL); carcinoembryonic antigen, 1.23 ng/mL (0–3 ng/mL); and carbohydrate antigen (CA) 19-9, 7.40 U/mL (0–37 U/mL). Abdominal computed tomography (CT) showed a well-defined hypodense mass located at the pancreas head and involving the stomach, as well as enlargement of several mesenteric and perigastric lymph nodes (Fig. 1). Upper gastrointestinal endoscopy revealed multiple geographic ulcers on the gastric body and antrum; therefore, histological examination was performed (Fig. 2). Endoscopic ultrasound-guided fine-needle aspiration and biopsy of a mass on the head of the pancreas were then performed using a 20-gauge needle (Echotip Procore®; Wilson-Cook, Cook Ireland Ltd., Limerick, Ireland). Histology of the pancreatic mass showed diffuse cellular infiltrates (Fig. 3A); atypical tumor cells were singly dispersed and bearing large round nuclei with prominent nucleoli (Fig. 3B). Large atypical cells were identified as CD20+ mature B cells (Fig. 3C). Histology of the gastric ulcers showed dense infiltration of the lamina propria with epithelial tenting (Fig. 4A). The tumor cells were oval to round with prominent nucleoli and opened chromatins (Fig. 4B). The results of immunohistochemical staining were as follows: CD20+ (Fig. 4C), CD3- (Fig. 4D), Bcl-2+ (Fig. 4E), MUM1+ (Fig. 4F), high proliferation index (Ki-67) (Fig. 4G), and CD30+ (Fig. 4H). Based on these histological features and immunophenotypes, a diagnosis of pancreatic diffuse large B-cell lymphoma involving the stomach was made. Bone marrow aspiration and biopsy samples were negative for lymphoma cell infiltration. At the time of writing, the patient had undergone six cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) with no complications. Comparison of positron emission tomography-CT findings before and after treatment showed a complete metabolic response (Fig. 5).
DISCUSSION
The pancreas is a rare extranodal site of non-Hodgkin lymphoma; the most common histological subtype of PPL is diffuse large B-cell lymphoma, which is present in 80% of patients with PPL [3,5]. PPL is generally diagnosed when the bulk of the disease is localized to the pancreas, with adjacent lymph node involvement and potentially distant spread [6]. Dawson et al. established diagnostic criteria for PPL that Behrns et al. have since modified [6-8]. The modified criteria include absence of palpable superficial lymphadenopathy, absence of mediastinal lymph node enlargement on chest radiography, normal leukocyte levels, a pancreatic mass observed in surgery with lymph node involvement confined to the pancreas, and no hepatic or splenic involvement [6,7]. Our patient exhibited a large mass on the pancreas head, diffuse wall thickening in the stomach, and adjacent lymph node enlargement on CT scan. A diagnosis of PPL was made based on imaging examinations, then confirmed by biopsy. PPL can involve organs other than the liver and spleen, as noted in the diagnostic criteria [7]. Two patients with PPL involving other organs have been described in South Korea [9,10]. One patient had PPL with kidney involvement and the other had PPL that externally compressed the common bile duct. However, no patient has been described with PPL involving the stomach. When lymphoma involves multiple organs, the primary site cannot be differentiated by pathologic findings or immunohistochemical staining [2]. We defined the primary lesion as the largest mass. In our patient, the large mass was predominantly located in the pancreas and was distinct from the gastric wall. Furthermore, pancreatic duct dilatation and mediastinal lymphadenopathy were not observed.
There is no specific clinical manifestation suggestive of PPL [1]. The most common symptoms are epigastric pain, a palpable abdominal mass, and weight loss [1-3,5]. Jaundice is infrequent in patients with PPL, despite a large mass at the head of the pancreas [6]. Symptoms often observed in patients with other lymphomas (e.g., fever, night sweats, and weight loss) are rarely present in patients with PPL [5]. Our patient complained of epigastric pain and weight loss, commonly associated with lymphomas.
Because laboratory test results are nonspecific, they do not aid the diagnosis of PPL [1]. The serum CA19-9 level is normal or slightly elevated in patients with PPL, unless biliary obstruction is present, whereas 80% of patients with pancreatic adenocarcinoma have a high CA19-9 level [1]. The serum lactate dehydrogenase and β2-microglobulin levels are often high in patients with lymphoproliferative diseases (e.g., lymphoma), but they are inevitably not increased in patients with PPL; moreover, elevated lactate dehydrogenase and β2-microglobulin levels are not required for the diagnosis of PPL [1,5]. However, higher lactate dehydrogenase and β2-microglobulin levels are indicators of a poor prognosis for patients with PPL [5]. Our patient had no specific findings in laboratory tests; his CA19-9 and lactate dehydrogenase levels were within normal ranges.
Contrast-enhanced CT is the imaging modality of choice for evaluation of a pancreatic mass [11]. PPL can be divided into two typical morphological groups on CT: 1) a localized, well-circumscribed tumoral form, and 2) a diffuse enlargement infiltrating or replacing most of the pancreatic gland. As in our patient, a bulky localized tumor in the pancreatic head without significant main pancreatic duct dilatation and with significant lymphadenopathy below the level of the renal veins, suggestive of PPL, is observed by CT [11]. Endoscopic ultrasound has also been widely used to evaluate pancreatic masses and surrounding structures; it is more sensitive than CT for detection of small lesions [1]. Furthermore, tissue sampling can be performed by endoscopic ultrasound-guided fine-needle aspiration or by biopsy with high sensitivity and specificity [4]. Positron emission tomography is helpful in the staging of lymphoma, differentiation of malignant from benign lesions, and evaluation of therapeutic response [11]. Although radiological examinations can provide support in the diagnosis of PPL, they are unable to distinguish between PPL and other pancreatic lesions. Therefore, clinicians must obtain cytohistological evidence for a definitive diagnosis of PPL [1].
The treatment strategy for PPL consists of surgery, radiotherapy, chemotherapy, or a combination thereof [12]. The role of surgery is controversial; aggressive surgical approaches that include pancreatic resection or tumor debulking are not preferential to chemotherapy because of the risk of surgical complications [3]. Surgical resection may play a role in the treatment of localized pancreatic lymphoma [12]. The role of radiotherapy also remains undefined; it is considered an adjuvant to chemotherapy [3]. The majority of patients with PPL receive chemotherapy alone [1,12]. The first-line chemotherapy regimen is prednisolone-vincristine-doxorubicin-cyclophosphamide (CHOP) [12]. CHOP chemotherapy in combination with rituximab (R-CHOP) was recently used for treatment of a patient with DLBCL that exhibited CD20 positivity [1]. Our patient’s tumor was positive for CD20 by immunohistochemical staining; thus, he underwent R-CHOP chemotherapy.
In conclusion, we present a rare instance of PPL involving the stomach, with symptoms including epigastric pain and weight loss. Diagnosis was made by endoscopic ultrasound-fine-needle aspiration and biopsy. PPL is a rare neoplasm, but should be considered in the differential diagnosis of pancreatic tumors. Because PPL is potentially curable and has a much better prognosis than adenocarcinoma, a correct diagnosis based on histological examination is important. The treatment strategy for PPL is controversial, but combination chemotherapy may be optimal.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print






