 |
 |
| Korean J Med > Volume 95(3); 2020 > Article |
|
Abstract
Severe fever with thrombocytopenia syndrome (SFTS) caused by the SFTS virus (SFTSV), a novel <i>Phlebovirus</i>, is endemic to South Korea, central and northeastern China, and western Japan. SFTS poses a threat to public health because of its high mortality and secondary transmission. Ticks and domestic animals are hosts for SFTSV in endemic areas. There is no specific treatment for SFTS, and avoiding tick bites is the best way to prevent infection. Early therapeutic plasma exchange (TPE) is a rescue therapy in patients with rapidly progressive SFTS. Here, we present a patient with SFTS who was improving on TPE but died suddenly due to acute lung injury after TPE.
Severe fever with thrombocytopenia syndrome (SFTS), caused by a novel Phlebovirus, is an emerging zoonotic disease in South Korea, China, and Japan [1,2]. The mortality rate of SFTS is 5–40% [1,2]. From 2013 to 2017, 607 cases of SFTS and 127 SFTS-associated deaths (20.9%) were reported in South Korea [2,3]. Despite its increasing frequency and high mortality rate, there is no effective treatment for SFTS [1,2]. Trials have evaluated treatments for severe or rapidly progressive SFTS. Therapeutic plasma exchange (TPE) is a surrogate choice [1,4-8]. However, there are no standard guidelines for implementing TPE for SFTS, and parameters such as the exchange frequency, volume, and inclusion or exclusion criteria have not been standardized. We report a patient with rapidly progressive SFTS and underlying ischemic heart disease who died suddenly from an acute lung injury and pulmonary edema after TPE. The study was approved by the Institutional Review Board of Jeju National University Hospital (201807014).
A 71-year-old man presented with a 6-day history of fever. He had a history of diabetes mellitus and had undergone stent implantation for acute myocardial infarction 15 days prior to presentation. He lived in a rural area and had been farming for 12 days since his discharge. On admission, he had chills, anorexia, and multiple tiny papular erythematous lesions, similar to insect bites, on both legs. However, he denied any history of tick bites. On hospital day 2, SFTS was diagnosed using real-time polymerase chain reaction; he had profound leukopenia and thrombocytopenia and was transferred to the intensive care unit to start TPE (Table 1).
On intensive care unit admission, he had a temperature of 38.3℃, blood pressure of 106/65 mmHg, pulse rate of 80 beats/min, acute physiology and chronic health evaluation II score of 8, and Glasgow Coma Scale score of 14. Laboratory tests revealed a platelet count of 40 × 103/µL (reference range [150–450] × 103/µL), white blood cell count of 1,500/µL (reference range 4,000–10,000/µL), activated partial thromboplastin time of 47 seconds (reference range 20.0–36.0 seconds), aspartate aminotransferase level of 72 IU/L (reference range 8–38 IU/L), alanine aminotransferase level of 29 IU/L (reference range 4–44 IU/L), and lactate dehydrogenase level of 686 IU/L (reference range 180–460 IU/L). Initially, he was treated with intravenous fluids and antibiotics (cefepime and azithromycin). TPE was initiated on hospital day 2 using the COBE® Spectra Apheresis System (Terumo BCT, Lakewood, USA). Acid citrate dextrose was used as the anticoagulant fluid, and the target exchange volume was one volume of body plasma. The plasma volume was calculated daily according to the patient’s weight and hematocrit level. Removed plasma was replaced with fresh frozen plasma (FFP), as conducted in previous patients [4,5]. On hospital day 3, the patient was tolerating all treatments, and on the morning of day 4, he stated that he was generally feeling better and wanted food. His body temperature was 37.8℃, and laboratory tests revealed a platelet count of 50 × 103/µL, white blood cell count of 2,000/µL, aspartate aminotransferase level of 84 IU/L, and alanine aminotransferase level of 25 IU/L (Table 1). With these improvements, we planned to transfer the patient to a general ward after completing three cycles of TPE. A routine morning chest X-ray was normal. The TPE was performed as usual. However, 30 minutes later, the patient suddenly became agitated, complaining of general discomfort and dyspnea and showed signs of respiratory distress (tachypnea) and significant arterial desaturation. Physical examination and chest X-ray revealed acute pulmonary edema (Fig. 1). TPE was immediately discontinued. Oxygen was supplied and intravenous diuretics were injected. Electrocardiography (ECG) showed no pathological wave form. The patient’s condition deteriorated rapidly, and cardiac arrest occurred. Cardiopulmonary resuscitation was started immediately, and endotracheal intubation was performed. Intravenous epinephrine and corticosteroids were administered for acute anaphylaxis and intravenous calcium gluconate for citrate-induced hypocalcemia. Spontaneous circulation returned after 1 minute of resuscitation. There were no abnormal laboratory findings, including ECG results and levels of cardiac markers, electrolytes, and acute phase reactants. Fig. 1 summarizes the patient’s clinical course. Unfortunately, he died despite repeated cardiopulmonary resuscitation efforts.
In Jeju National University Hospital, TPE has been the treatment of choice for rapidly progressive SFTS since 2013, as it decreases serum cytokine levels and viral loads in patients with SFTS, with eventual improvement in clinical parameters [4,5]. Between 2013 and September 2018, 18 of 52 SFTS patients underwent TPE at Jeju National University Hospital, with no fatal adverse effects of TPE, including shock and multi-organ failure, during the treatment of rapidly progressive SFTS.
Plasma exchange involves separation of all cellular elements of the blood using an extracorporeal semipermeable membrane [9]. Although plasma exchange is generally considered safe, there have been reports of thrombosis as an adverse event [10]. A review of reported complications associated with 15,658 TPE treatments found that serious complications were uncommon and included cardiovascular events (0.2%), respiratory events (0.2%), anaphylactic reactions (0.25%), and death (0.05%) [9]. TPE involves the replacement of intravascular plasma with 5% albumin, FFP, or a mixture of the two. Adverse reactions are more commonly associated with the administration of FFP, which exposes patients to the risks associated with transfusion [9]. Transfusion-related acute lung injury (TRALI) is the most common cause of death from blood transfusion. The plasma components suggest lung injury, but this issue needs further study. TRALI is associated with predisposing conditions such as major surgery, active bacterial or viral infection, and massive transfusion [11]. Most TRALI events occur within the first 2 hours after transfusion. Our patient had a history of acute myocardial infarction and underwent coronary angioplasty with stent implantation 15 days earlier. He also received daily transfusions of 15 U FFP for 2 consecutive days for fluid replacement during TPE. Therefore, we suspected that his death was caused by TRALI.
The patient may have developed acute pulmonary edema due to acute ischemic heart disease, considering his history of acute myocardial infarction. However, he did not show evidence of cardiogenic causes on ECG or cardiac marker analysis after resuscitation. The most common cause of pulmonary edema is increased microvascular hydrostatic pressure and increased capillary permeability [12]. Because he received a large volume of fluids, TPE might have aggravated the pulmonary edema.
The cause of the patient’s death is unclear, but TPE was considered the cause of the acute pulmonary edema and rapid deterioration of his condition. Fourteen days before his admission for SFTS, transthoracic echocardiography revealed an ejection fraction of 61.2% and minimal to mild aortic regurgitation, suggesting good cardiovascular function despite stent implantation. He died after approximately 30 minutes of respiratory distress. Because his death was unexpected, no procedures that could detect the cause of acute lung injury, such as chest computed tomography or transthoracic echocardiography, were performed.
As a treatment for SFTS, TPE is controversial, and the optimal duration and dose of FFP have not been determined. In South Korea, clinicians tend to use TPE to treat SFTS, but the efficacy of TPE in this setting awaits the results of controlled trials, which are currently underway. Moreover, because pulmonary edema caused by acute lung injury is a critical adverse effect of TPE, physicians should consider the possible complications associated with this procedure for treating SFTS. In conclusion, the use of TPE to treat SFTS should be decided carefully, along with the TPE frequency and replacement fluid, especially in patients with recent ischemic heart disease.
REFERENCES
1. Liu Q, He B, Huang SY, Wei F, Zhu XQ. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect Dis 2014;14:763–772.


2. Yu XJ, Liang MF, Zhang SY. al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med 2011;364:1523–1532.



3. Li H, Lu QB, Xing B, et al. Epidemiological and clinical features of laboratory-diagnosed severe fever with thrombocytopenia syndrome in China, 2011-17: a prospective observational study. Lancet Infect Dis 2018;18:1127–1137.


4. Oh WS, Heo ST, Kim SH, et al. Plasma exchange and ribavirin for rapidly progressive severe fever with thrombocytopenia syndrome. Int J Infect Dis 2014;18:84–86.


5. Oh WS, Yoo JR, Kwon KT, et al. Effect of early plasma exchange on survival in patients with severe fever with thrombocytopenia syndrome: a multicenter study. Yonsei Med J 2017;58:867–871.



6. Park I, Kim HI, Kwon KT. Two treatment cases of severe fever and thrombocytopenia syndrome with oral ribavirin and plasma exchange. Infect Chemother 2017;49:72–77.



7. Kim UJ, Kim DM, Ahn JH, et al. Successful treatment of rapidly progressing severe fever with thrombocytopenia syndrome with neurological complications using intravenous immunoglobulin and corticosteroid. Antivir Ther 2017;21:637–640.

8. Yoo JR, Kim SH, Kim YR, Lee KH, Oh WS, Heo ST. Application of therapeutic plasma exchange in patients having severe fever with thrombocytopenia syndrome. Korean J Intern Med 2019;34:902–909.


9. Mokrzycki MH, Kaplan AA. Therapeutic plasma exchange: complications and management. Am J Kidney Dis 1994;23:817–827.


10. Salahuddin H, Sheikh AA, Hussaini S, Verghese C, Tietjen GE. Ischemic stroke after plasmapheresis. Am J Med Sci 2018;356:399–403.


The clinical course of a patient with severe fever with thrombocytopenia syndrome and chest X-ray changes. (A) Nonspecific findings in the lung parenchyma before therapeutic plasma exchange (TPE) and (B) diffuse peripheral infiltration in both lungs after 30 minutes of TPE on hospital day 4. ER, emergency department; PE, plasma exchange; CPR, cardiopulmonary resuscitation.
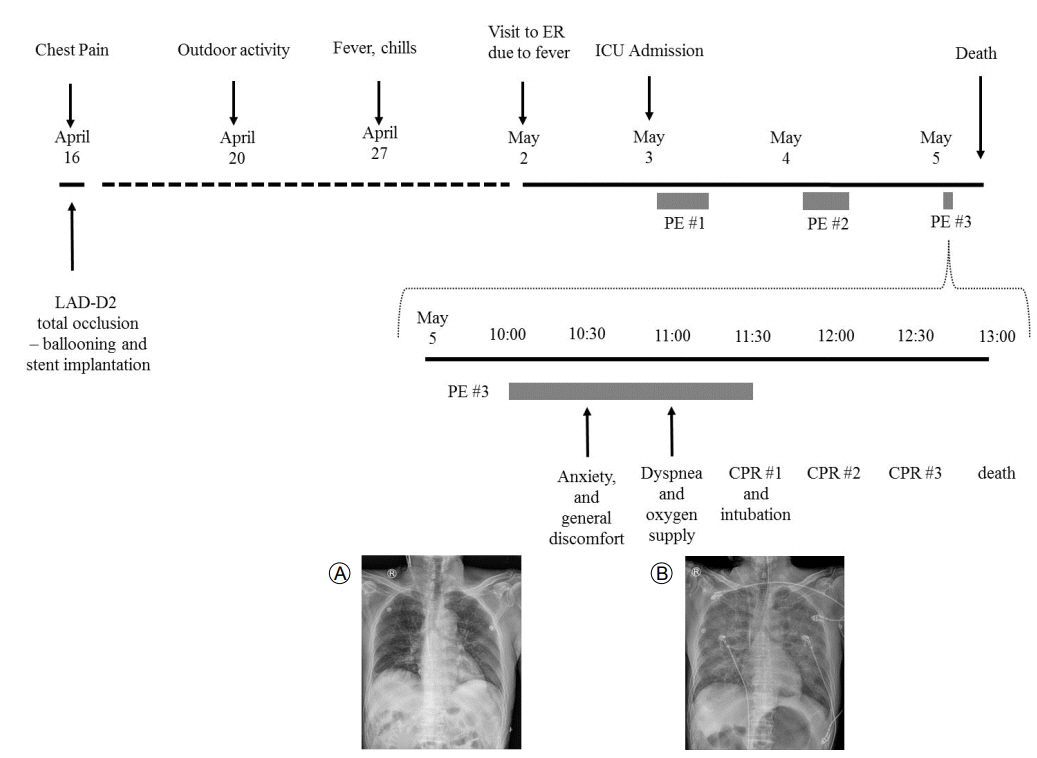
Figure 1.
Table 1.
Clinical course of the patient with severe fever with thrombocytopenia syndrome
| HD 1 | HD 2 | HD 3 | HD 4a | |
|---|---|---|---|---|
| Respiratory symptoms | No | No | No | No |
| Body temperature (℃) | 38.1 | 38.3 | 38.6 | 37.8 |
| WBC (/μL) | 2,300 | 1,500 | 1,800 | 2,000 |
| Neutrophil count (%) | 57.2 | 51.4 | 48.3 | 49.5 |
| Platelet count (×103/μL) | 66 | 40 | 27 | 50 |
| AST (IU/L) | 68 | 72 | 91 | 84 |
| ALT (IU/L) | 33 | 29 | 29 | 25 |
| Creatinine (mg/dL) | 0.94 | 1.0 | N/A | 0.65 |
| GCS | 14 | 14 | 14 | 4 |
| MODs | 2 | 2 | 2 | 2 |
| ECG | Nonspecific changes | N/A | N/A | Nonspecific changes |
| Chest X-ray | No active lung lesion | N/A | No active lung lesion | No active lung lesion |
| TPE | No | Yes | Yes | Yes |



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print




