 |
 |
| Korean J Med > Volume 91(3); 2016 > Article |
|
Abstract
Here we describe a case of rapidly expanding ascending aortic aneurysm in a patient with relapsing polychondritis. To prevent aneurysm rupture, the patient underwent emergent surgical repair. Silent inflammation can progress in the aorta wall, even in asymptomatic patients with mild disease activity under immunosuppressive treatment, leading to the rapid growth of aortic aneurysms. Close monitoring with routine imaging is needed once a patient with relapsing polychondritis is diagnosed with an aortic aneurysm.
Relapsing polychondritis is a rare multi-systemic disease affecting cartilaginous structures and other connective tissues, including the ears, nose, trachea, vessels, eyes, and joints. The average age of patients at the time of diagnosis is between 40 and 50 years, and men and women are affected equally. Although previous studies have indicated that there is some genetic susceptibility to the disease, it is not a familial condition [1]. The pathogenesis of relapsing polychondritis remains unclear, but both cellular and humoral immune reactions to type II collagen seem to play a role in genetically susceptible patients with human leukocyte antigen-DR4 [2].
Cardiovascular involvement is a frequent manifestation of this disease, and it leads to significant mortality. Vascular disease has been observed in 16% of patients, the most common manifestation being aortic disease, which occurs in 5 to 7% of patients [3]. Pearson et al. [4] first described an aortic aneurysm in 1967, and it has since been recognized as a relatively frequent complication of relapsing polychondritis. Aneurysms occur more frequently in the ascending aorta; however, patients may have multiple aneurysms involving both the thoracic and abdominal aorta. Ascending aortic aneurysms commonly lead to aortic insufficiency. Sudden deaths reported in the literature may be due to aortic aneurysm rupture [5].
Here we report a case of relapsing polychondritis with a rapidly expanding ascending aortic aneurysm that required emergency surgery due to the risk of rupture.
A 28-year-old male was diagnosed with relapsing polychondritis due to right ear chondritis at another hospital. About a year before the diagnosis, he experienced minor right eye trauma causing scleritis, which lasted for over a year even with topical steroid treatment. The ophthalmologist suspected an unknown autoimmune disease to be the cause of persistent inflammation of the sclera. A year after the eye trauma, right otalgia developed suddenly and the patient was admitted to the ear, nose, and throat ward after outpatient treatment with oral antibiotics failed to improve his symptoms. After a week of unsuccessful treatment with intravenous antibiotics and pain killers, the patient was transferred to the rheumatology department; a right ear biopsy confirmed relapsing polychondritis. Intravenous steroid treatment rapidly relieved the patientвҖҷs right ear chondritis, and he was discharged with an oral steroid and a plan to taper the dose on an outpatient basis.
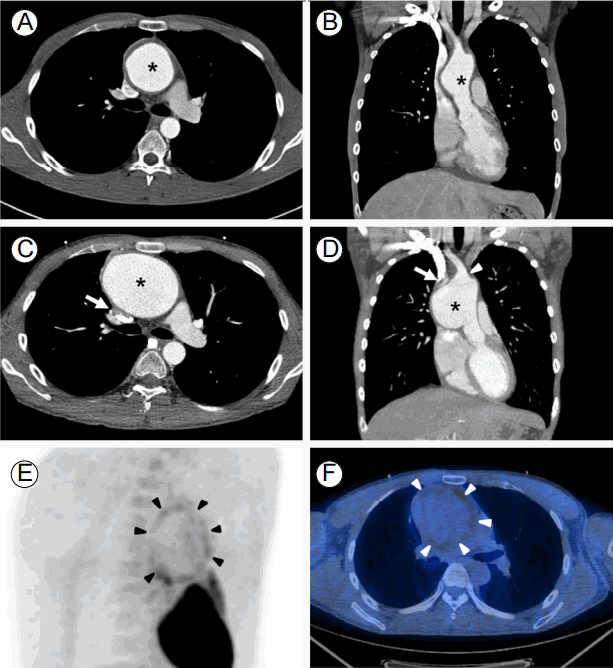
Several months of outpatient follow-up visits went on uneventfully, and the oral steroid dose was reduced with careful monitoring of inflammatory markers. However, the patient complained of chest pain when coughing. Chest computed tomography (CT) revealed concentric wall thickening with a fusiform aneurysm of the ascending thoracic aorta measuring 56 mm in diameter (Fig. 1A and 1B).
The patient was referred to our hospital for surgical repair of the aortic aneurysm. Elective surgery was planned, and he underwent a routine work-up for open ascending aorta replacement. Although the patientвҖҷs erythrocyte sedimentation rate was moderately elevated at 55 mm/h (reference range, 0-22 mm/h), his C-reactive protein level was low at 0.3 mg/dL (reference range, 0-0.3 mg/dL). Assuming that inflammation due to relapsing polychondritis was under control, oral steroid therapy was continued with a plan to change to intravenous stress doses during admission for surgery. However, CT angiography revealed rapid growth of the aneurysm to 81 mm in a single month (Fig. 1C and 1D). Transthoracic echocardiography revealed an intact aortic root without aortic valve regurgitation. Fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT demonstrated increased uptake of the aneurysm wall, which raised suspicion of active aortitis (Fig. 1E and 1F). Except for the aortic aneurysm, no significant organ uptake was present in the FDG-PET/CT scan.
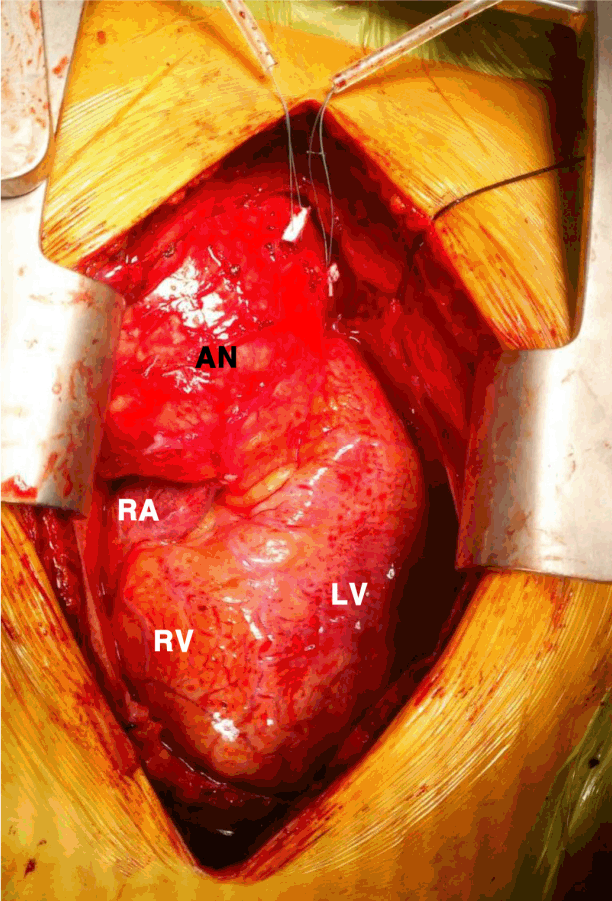
Emergent surgical repair was performed. Surgically, the aneurysm wall was adhered to the pericardium, which was filled with a bloody effusion. The wall of the aneurysm was extremely thin and easily torn, even with gentle manipulation. Vascular wall thickening was noted on the PET-positive aortic segments (Fig. 2).
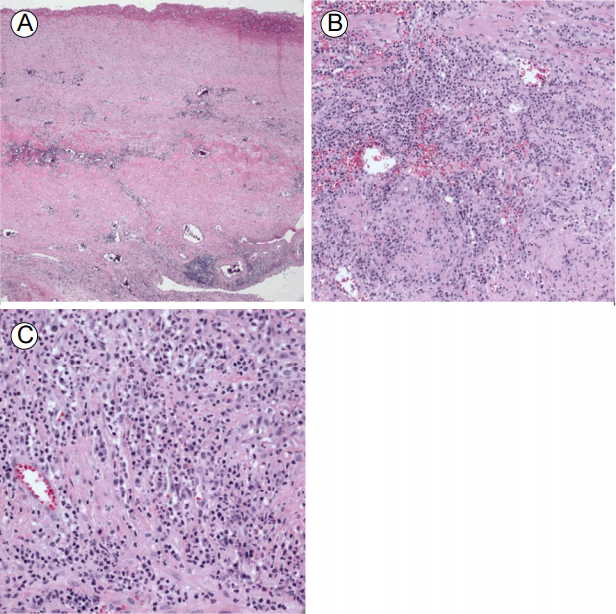
The pathologic findings were consistent with previously reported active aortitis in relapsing polychondritis [6]. On gross examination, the aorta was cystically dilated. The medial wall was mostly destroyed and replaced by mixed inflammatory cells consisting of lymphocytes, plasma cells, and neutrophils. Most of the medial elastic tissue was replaced with granulation tissue and focal areas of necrosis. Neutrophilic leukocyte aggregates forming microabscesses were present in the media and intima (Fig. 3).
The patientвҖҷs post-operative recovery was uneventful under steroid and azathioprine treatment.
Aortic aneurysm is a relatively frequent complication in patients with relapsing polychondritis; it was first reported in the 1960s. In Korea, there have been several case reports of relapsing polychondritis with various presentations, but none have described aortic aneurysms. There was one case of aortitis mimicking TakayasuвҖҷs arteritis without dilatation, which was treated medically [7]. The largest case series in Korea included 16 patients with relapsing polychondritis. Among the 16 patients, only one had cardiovascular involvement exhibiting aortitis, which is a considerably lower rate of cardiovascular involvement (6.25%) than seen in foreign case series. Due to the small number of cases, it was not possible to draw any firm conclusions about the rate of cardiovascular involvement in Korean patients with relapsing polychondritis [8].
In 2013, Jacobs et al. [9] described a case of thoracic aortic aneurysm associated with relapsing polychondritis repaired by a staged endovascular stent graft following open surgical repair. It was the first reported successful endovascular repair of an aortic aneurysm in relapsing polychondritis, and it showed that an endovascular approach is promising even in patients with relapsing polychondritis. On the other hand, our case demonstrates the importance of close monitoring of aortic aneurysms with imaging studies, even if medical treatment successfully suppresses inflammation. As shown in this case, low serum titers of inflammatory markers should not be the excuse to withhold routine follow-up imaging.
Aortic aneurysms in relapsing polychondritis occur secondary to active aortitis. Rich proteoglycan in the aorta wall is thought to be the target of autoimmune processes. Chronic inflammation of the vessel wall leads to a loss of elastic tissue and replacement with fibrous collagen [4]. Eventually, this leads to a loss of structural integrity of the vessel wall, presenting with rapid degeneration of the aortic aneurysm, which may result in fatal rupture. Silent inflammation may progress in the aortic wall even in asymptomatic patients with mild disease activity under immunosuppressive treatment. Thus, regular imaging of all aortic segments is strongly recommended in patients diagnosed with cardiovascular involvement [10].
However, the lack of awareness of this condition and the paucity of established guidelines regarding follow-up and the optimal timing of intervention may put these patients at risk of fatal complications.
Once a patient with relapsing polychondritis is diagnosed with an aortic aneurysm, careful monitoring and follow-up with routine imaging is needed to determine whether medical therapy is sufficient to control the disease.
This case illustrates the importance of close monitoring of aortic aneurysms in patients with relapsing polychondritis to prevent potentially fatal complications via timely detection and surgical intervention.
REFERENCES
1. Zeuner M, Straub RH, Rauh G, Albert ED, SchГ¶lmerich J, Lang B. Relapsing polychondritis: clinical and immunogenetic analysis of 62 patients. J Rheumatol 1997;24:96вҖ“101.

2. Sharma A, Gnanapandithan K, Sharma K, Sharma S. Relapsing polychondritis: a review. Clin Rheumatol 2013;32:1575вҖ“1583.


3. Del Rosso A, Petix NR, Pratesi M, Bini A. Cardiovascular involvement in relapsing polychondritis. Semin Arthritis Rheum 1997;26:840вҖ“844.


4. Pearson CM, Kroening R, Verity MA, Getzen JH. Aortic insufficiency and aortic aneurysm in relapsing polychondritis. Trans Assoc Am Physicians 1967;80:71вҖ“90.

5. Michet CJ Jr, McKenna CH, Luthra HS, O'Fallon WM. Relapsing polychondritis. Survival and predictive role of early disease manifestations. Ann Intern Med 1986;104:74вҖ“78.


6. Stone JR, Bruneval P, Angelini A, et al. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association for European Cardiovascular Pathology: I. Inflammatory diseases. Cardiovasc Pathol 2015;24:267вҖ“278.


7. Park JJ, Lee JC, Choi HJ, et al. A case of relapsing polychondritis with vasculitis mimicking Takayasu's arteritis. Korean J Med 2004;67(Suppl 3):S732вҖ“S735.
8. Park JJ, Lee JC, Kim JH, et al. Clinical analysis of relapsing polychondritis -16 cases in Korea-. J Rheum Dis 2005;12:213вҖ“221.
Fusiform aneurysm (*) of the ascending thoracic aorta with concentric wall thickening measuring 56 mm in diameter (A and B). Rapid growth of the aneurysm in a single month. Aneurysm arising from 1.7 cm above the sinotubular junction extending to the origin of the right innominate artery (arrowhead in D). Note the large aneurysm causing extrinsic compression of the superior vena cava (arrows in C and D). This aneurysm was 81 mm in the orthogonal plane (C and D). FDG-PET/CT demonstrating mild diffuse FDG uptake (SUVmax = 2.9) along the aneurysm wall (arrowheads) (E and F). FDG-PET/CT, fluorodeoxyglucose-positron emission tomography/computed tomography; SUV, standardized uptake value.
FigureВ 1.
Intraoperative image of the ascending aortic aneurysm. AN, aneurysm; RA, right atrium; RV, right ventricle; LV, left ventricle.
FigureВ 2.
Low-power microscopic examination showing destruction of the normal intima and media structure (A: hematoxylin and eosin [H&E], Г—40). The aortic media was infiltrated with mixed inflammatory cells and granulation tissue (B: H&E, Г—200). High-power microscopy showing areas of focal microabscess (C: H&E, Г—400).



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print




