INTRODUCTION
Hypertrophic cardiomyopathy (HCM) is usually considered a disease of the left ventricle (LV). Although concurrent right ventricular involvement is not uncommon, significant right ventricular outflow tract (RVOT) obstruction due to a hypertrophied right ventricle (RV) is very rare [1]. This report presents a rare and unique phenotype of HCM affecting both ventricles and causing severe RVOT obstruction.
CASE REPORT
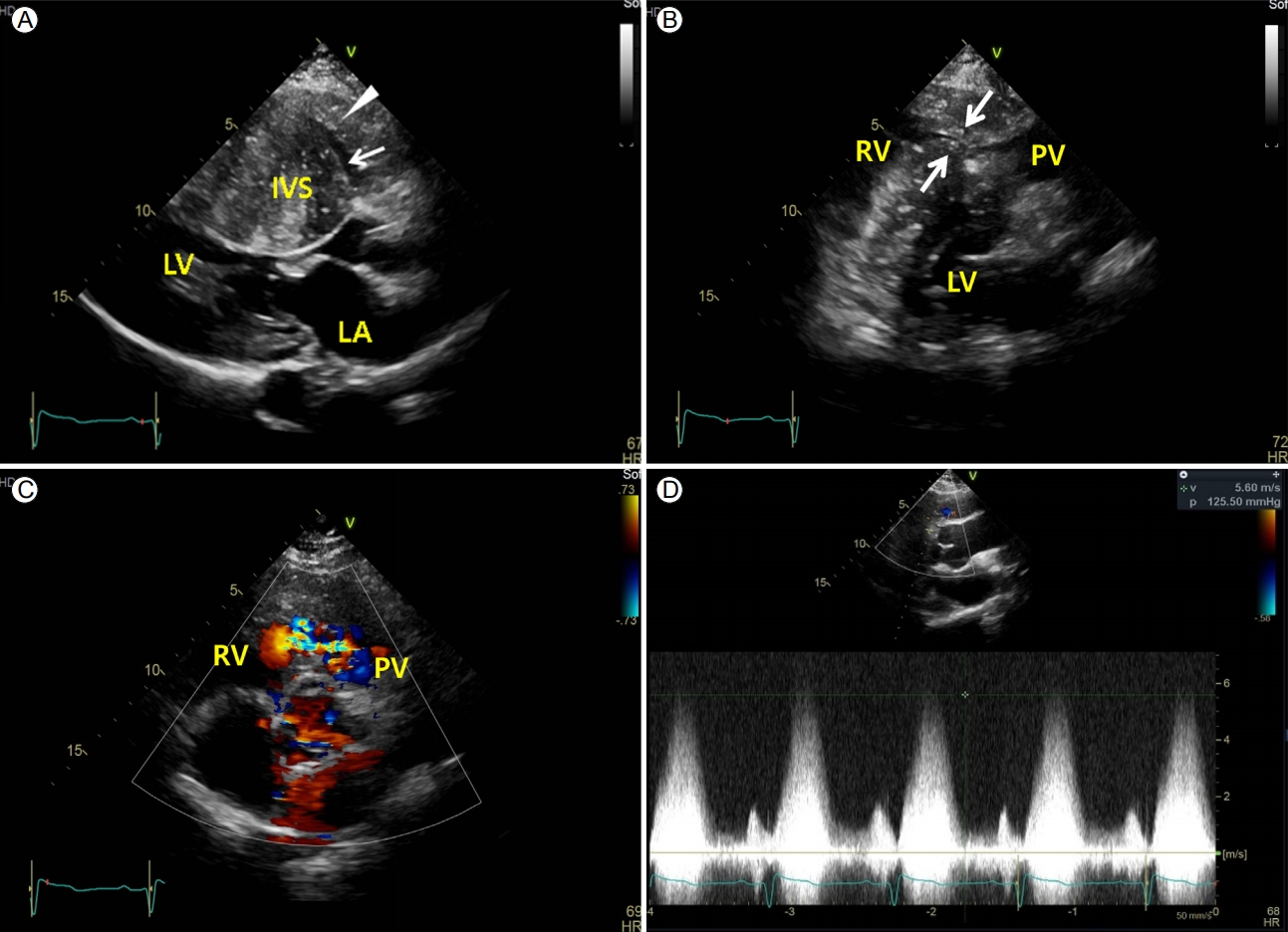
A 29-year-old male patient without family history of HCM or any known heart condition was admitted with progressive worsening dyspnea of New York Heart Association (NYHA) class Ōģó. He was well developed, with a height and weight of 172 cm and 63 kg, respectively, and did not have abnormal external facial or body features. Physical examination at the time of admission revealed blood pressure and heart rate of 144/101 mmHg and 81 beats/min, respectively. A grade Ōģó systolic ejection murmur was detected on the left upper sternal border. Chest radiography revealed cardiomegaly (Fig. 1A). Electrocardiography demonstrated sinus rhythm; left axis deviation; right atrial enlargement; right bundle branch block; T-wave inversions on leads I, aVL, and V1-V6; and positive R wave on lead aVR (Fig. 1B). Frequent ventricular premature contractions (VPCs) and short runs of non-sustained ventricular tachycardia (NSVT) were noted on Holter monitoring. His laboratory results revealed oxygen saturation of 99% in room air, NT-pro brain natriuretic peptide level of 6,133 pg/mL (0-125), troponin T level of 0.053 ng/mL (0-0.014), creatine kinase-myocardial band (CK-MB) level of 3.4 ng/mL (< 4.87), serum kappa light chain level of 15.1 mg/L (3.30-19.40), serum lambda light chain level of 10.88 mg/L (5.71-26.30), transthyretin gene mutation (-), Fabry disease screening (-), alpha-galactosidase A gene (Fabry) mutation (ŌłÆ), 24-hour urinary metanephrine level of 108.4 ╬╝g/day (52.0-341.0), 24-hour urinary normetanephrine level of 209.8 ╬╝g/day (88.0-444.0), epinephrine level of 12.0 ╬╝g/day (0-20), norepinephrine level of 54.2 ╬╝g/day (15-80), plasma metanephrine level of 0.26 nmol/L (< 0.5), plasma normetanephrine level of 0.31 nmol/L (< 0.9), plasma renin activity of 3.02 ng/mL/h, and serum aldosterone level of 17.0 ng/dL. The ankle brachial index was 1.1 at the right ankle and 1.2 at the left ankle. Coronary arteriography revealed normal findings. Echocardiography demonstrated diffusely and severely hypertrophied LV (particularly severe interventricular septal hypertrophy, mid-septal thickness = 40 mm, apical lateral thickness = 34 mm) with small LV cavity and a small left ventricular outflow tract (LVOT) area with dynamic obstruction (resting systolic peak pressure gradient, 42 mmHg) (Fig. 2A). The RV was diffusely hypertrophied (RV free wall thickness, 15 mm). The RV infundibulum was severely hypertrophied and narrowed with significant systolic RVOT flow acceleration (resting systolic peak pressure gradient, 125 mmHg) (Fig. 2B-2D). No pulmonary stenosis was observed but mild pulmonary valvular regurgitation was detected. Cardiac computed tomography scan showed severe LV hypertrophy, RV hypertrophy with trabecula septomarginalis (TSM), and moderate band hypertrophy (Fig. 3A), which resembled double-chambered RV. The LVOT was also stenotic due to basal septal bulging. Cardiac magnetic resonance imaging also showed diffusely and markedly thickened myocardium of the LV and RV with prominent TSM and moderate band thickening. Late gadolinium enhancement (LGE) imaging revealed multifocal nodular heterogenous delayed enhancement at the thickened myocardium involving the RV free wall, suggesting multiple areas of myocardial fibrosis; the LGE extent values of the LV and RV were 6% and 13%, respectively (Fig. 3B). Because the patientŌĆÖs subjective symptoms and objective findings were severe enough and the symptoms had not improved with medical treatment, surgery was performed. The double-chambered right ventricle muscle and subaortic fibromuscular LV septum were surgically resected. A pathological examination revealed hypertrophied muscle bundles with fibrosis (Fig. 3C). Immediately after surgery, the patient was hemodynamically stable, and the VPCs and short runs of NSVT rhythm stabilized. Postoperative echocardiography showed widened RVOT and LVOT areas, with a resting RVOT systolic peak pressure gradient of 30 mmHg (Fig. 4) and resting LVOT systolic peak pressure gradient of 25 mmHg. The patientŌĆÖs dyspnea improved to NYHA classification 0-I with only small dose of a beta blocker.
DISCUSSION
HCM is recognized as genetic predisposition to myocardial hypertrophy and has diverse clinical and pathological features according to the extent and severity of hypertrophy. HCM can cause heart failure, arrhythmia, thromboembolism, and sudden cardiac death. Hypertrophy may cause an LVOT obstruction and hemodynamic instability when it involves the LV septum. RV hypertrophy in a patient with HCM is not uncommon, but a concomitant RVOT obstruction is rare in biventricular HCM, as in this case. Some cases of severe RVOT obstruction in patients with biventricular hypertrophy have been reported in children with genetic syndromes, such as Noonan syndrome.
When this patient first underwent echocardiography, the hypertrophy of both ventricles was very severe and both ventricular cavities were very small, making it difficult to identify the disease entity compared with the commonly seen cardiac diseases on routine echocardiography. As reported previously [2], the relatively limited spatial resolution of the RV and non-parallel Doppler angle for RVOT interrogation restricted the ability to assess the RV anatomy and hemodynamics to diagnose an associated RVOT obstruction in this patient with HCM and an LVOT obstruction.
We needed to differentiate other diseases causing hypertrophy, such as infiltrative diseases, storage diseases, and other systemic cardiac diseases. We could not find any association between these diseases and the clinical findings and laboratory tests, nor did we find any syndromes based on the clinical features. We thought of this as a case of HCM and could confirm when the postoperative biopsy of the resected muscle revealed findings consistent with HCM.
A beta blocker was used empirically, but it did not result in any subjective or objective improvement of symptoms in this patient. Some clinicians believe that this severe form of HCM with an RVOT obstruction is best treated by transplantation, in contrast to the good long-term effect of surgical myectomy in patients with a dynamic LVOT obstruction [3] when symptoms are intolerant or unresponsive to medical treatment. Heart transplantation and surgical repair are controversial for these patients. Limited data are available on the surgical management of this condition, and there is no standardized technique. Borisov [4] reported a surgical technique in a cohort of seven patients with a single limited RV longitudinal incision. Quintana et al. [5] advocated the biventricular cavity approach to relieve an obstruction from both LVOT and RVOT and suggested that conventional surgery should be offered in the case of a biventricular obstruction in a patient with HCM. Because the RVOT obstruction was morphologically and hemodynamically severe and the patientŌĆÖs symptoms were refractory to medication, other invasive measures were considered in the present case. We thought that, anatomically, the hypertrophied muscles (particularly the RVOT muscles) could possibly be resected and the obstruction can be relieved by this surgical method, so we decided to perform a conventional operation to resect the hypertrophied muscles of the RVOT before considering other methods, such as cardiac transplantation. The severely hypertrophied LVOT muscle was concomitantly resected as well due to severe hypertrophy even with moderate hemodynamic significance through the LVOT. Although the patientŌĆÖs symptoms and hemodynamic parameters improved dramatically, the remaining problems of systolic and diastolic heart failure associated with remnant hypertrophy, critical arrhythmia, and the risk of thromboembolism should be continuously monitored during postoperative medical care.
In conclusion, we hereby report a case of a 29-year-old male patient with a severe RVOT obstruction and biventricular HCM. The HCM was non-syndromic, and the patient did not have family history of cardiac disease. The RVOT and LVOT muscles were concomitantly resected successfully, and is now being followed with small dose of a beta blocker.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print






