INTRODUCTION
Amyloidosis develops in 10-15% of patients with light-chain myeloma [1] and involves mainly the kidney, heart, skin, gastrointestinal tract, and nervous system due to light-chain organ tropism, other organs may be involved less frequently [2,3]. Multi-organ involvement is a very poor prognostic sign, particularly if the heart and bowel are affected [4]. Once the disease becomes symptomatic, it rapidly becomes progressive and usually becomes fatal, with median survival duration of 12-18 months. Fewer than 5% of patients survive for 10 years or longer [5]. However, the course of amyloidosis is difficult to document because determining the time of orgin of the disease is seldom possible [1].
Pericardial involvement is a rare complication of multiple myeloma, occurring in < 1% of patients and usually manifests at a late stage of the disease [6,7]. A cardiac amyloidosis is not rare; however, deposit of amyloid in the pericardium without the involvement of the myocardium is extremely rare. Here, we report a rare case of pericardial amyloidosis that was associated with light-chain myeloma, without an evidence of myocardial involvement.
CASE REPORT
A 66-year-old female was admitted to our hospital with dyspnea that had persisted for 2 months. She had no history of dyspnea until this episode. The dyspnea developed progressively and led to breathing difficulties. She had pleuritic chest pain for 2 weeks that was aggravated by deep inspiration. Her vital signs were: blood pressure, 107/56 mmHg; body temperature, 36.2℃; pulse rate. 92/min; and respiratory rate, 18/min. A chest X-ray showed no abnormalities. Hemoglobin, hematocrit, and reticulocyte count were 7.2 g/dL, 21.7%, and 1.8%, respectively. White blood cell and platelet counts were 6,920/μL and 252,000/μL, respectively. Total serum protein was 5.8 g/dL, serum albumin was 3.6 g/dL, and total serum calcium was 9.4 mg/dL. A peripheral blood smear showed normocytic normochromic anemia with anisopoikilocytosis and mild polychromasia, as well as mild neutrophilia with slight toxic granulation. Electrocardiography revealed a normal sinus rhythm, a normal voltage amplitude and a T-wave inversion on leads II, III, and aVf. The patient was transfused with two units of packed red blood cells because she was suffering from severe dyspnea. Despite the transfusion, her dyspnea recurred 2 weeks later. Cardiomegaly and bilateral pleural effusion developed during the 2-week follow-up period. Echocardiography revealed normal systolic function with a 61% ejection fraction. However, there was pericardial effusion with no myocardial thickening (1.2 cm on the posterior side of the posterior four-chambered view, 1.3 cm on the RA side of the anterior four-chambered view). A chest computed tomography scan showed bilateral pleural effusion and adjacent both lower lungs and right-middle lung atelectasis, cardiomegaly,and mild interstitial pulmonary edema. No evidence of pulmonary thromboembolism was observed, and coronary angiography showed no evidence of ischemic cardiomyopathy. A pericardiopleural window operation was performed immediately. A histological examination of the pericardium showed homogeneous eosinophilic deposits around the adipocytes (Fig. 1A). Congo-red staining was positive and appeared as a bright greenish birefringency under a polarized light microscope (Fig. 1B). An electron microscopic examination revealed amyloids in collections of 6-10 nm nonbranched, straight, slightly bent, or rigid-looking fibrils (Fig. 1C).
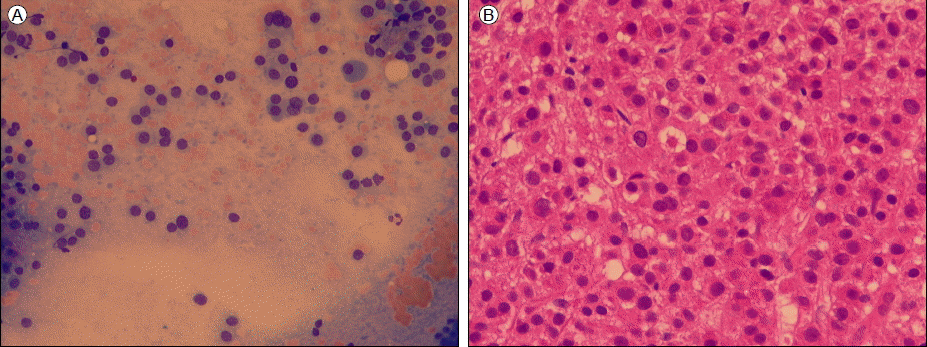
A skull X-ray showed osteolytic lesions, but no lesions were detected in other bones. Serum electrophoresis (EP) and immunofixation (IF) revealed a lambda-type monoclonal gammopathy. Urine EP and IF also showed a lambda type monoclonal gammopathy, and light-chain immunoglobulin was excreted at 1.01 g/day in urine. An aspirated specimen of the bone marrow showed 67.2% plasma cell infiltration (Fig. 2). Serum β2-microglobulin was 2.69 mg/L.
Therefore, we diagnosed pericardial amyloidosis associated with light-chain myeloma. The patient was initially treated with dexamethasone and thalidomide. Chemotherapy was then switched to a regimen of oral mephalan and prednisolone (MP) because the patient was intolerant to thalidomide. She responded well to the MP regimen. The M-spike completely disappeared after three cycles of MP chemotherapy. The lambda light chain was also not precipitated in the immunofixation. Complete remission was sustained after six cycles of chemotherapy, and serum β2-microglobulin decreased to 2.28 mg/L after completion of chemotherapy. Remission was maintained for 1 year without chemotherapy; however, light-chain immunoglobulinuria (0.291 g/day) was detected after 1 year. Urine IF revealed a monoclonal gammopathy of the lambda type, and serum β2-microglobulin had increased to 2.60 mg/L, suggesting a recurrence of light-chain myeloma. She suffered from progressive dyspnea, even in a resting state. Echocardiography showed a thickening of the ventricular wall with granular sparkling on the myocardium, which supported a diagnosis of cardiac amyloidosis. Despite progressive congestive heart failure caused by amyloid infiltration of the myocardium, we decided to treat her symptomatically, without chemotherapy, due to her poor general condition.
DISCUSSION
The main constituent of amyloid deposits is the variable region of the light chains, which are deposited as fibrils throughout the body [4,5]. In amyloidosis associated with light-chain myeloma, the kappa light chain is the predominant form, with a ratio of 2:1 over the lambda light chain, such as observed in this patient. However, the ratio of the kappa light chain to the lambda chain is 1:2 in primary idiopathic amyloidosis [1,5].
In our case, the patient showed a restrictive pattern of heart failure with pericardial effusion at the time of diagnosis, even though her disease eventually progressed to involve the myocardium. The optimal treatment for malignant involvement of the pericardium in cases of myeloma is not yet known; thus, it is often associated with a poor prognosis, as seen in the cases in Table 1[6-8]. Among these cases, only two cases mentioned that myeloma was also involved in myocardium. Goldberg et al. [8] reported a case in which plasma cells infiltrated the myocardium. However, the case reported by Rosenbaum et al. [6] showed no involvement of plasma cells in the myocardium. Our patient showed a better prognosis and complete remission for 1 year even in the presence of pericardial amyloidosis. About 10 cases of multiple myeloma with pericardial involvement have been reported since 1966. [7]; pericardial amyloidosis in cases of multiple myeloma has been reported only rarely. There are several reports of light-chain amyloidosis involving rare sites such as the pleura [9].
Treatment for amyloidosis is directed both toward the affected organs and toward the specific disease. A patient with restrictive cardiomyopathy who exhibits signs of cardiac amyloidosis may be treated with diuretics. However, this treatment may worsen heart function after the administration of calcium channel blockers, digoxin, and β-blockers [4,10]. If a patient with multiple myeloma is < 65 years old and does not have severe co-morbidities, then a high dosage of intravenous mephalan (200 mg/m2), as induction chemotherapy, and autologous stem cell transplantation is recommended [11]. Several case reports have documented prolonged survival and resolution of heart failure or proteinuria in patients receiving oral MP. The active plasma cell dyscrasia generally disappears after chemotherapy in these cases, despite persistent amyloidosis in affected tissues [4].




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print






