 |
 |
| Korean J Med > Volume 98(5); 2023 > Article |
|
Abstract
Podocytic infolding glomerulopathy (PIG) is a rare disease diagnosed by its characteristic structure on electron microscopy. Histologically, there are microtubules and microspheres in the glomerular basement membrane (GBM). Most PIG has been reported in Japan, with a single case in South Korea; there are no previous reports of PIG in kidney transplant patients. Here, we report a 47-year-old Korean woman diagnosed with PIG after a kidney transplant. She was diagnosed with systemic lupus erythematosus. She was suspected of having lupus nephritis and subsequently underwent kidney transplantation. Routine testing showed increased proteinuria without renal functional impairment. A biopsy of the allograft kidney revealed mild interstitial fibrosis, tubular atrophy, and GBM thickening with intramembranous microspherules and microtubules on light microscopy. Electron microscopy showed GBM thickening with intramembranous microspherules and microtubules. These findings are consistent with PIG.
Podocytic infolding glomerulopathy (PIG) is a rare disease diagnosed by the presence of microtubules and microspheres in the glomerular basement membrane (GBM) on electron microscopy. PIG is frequently associated with autoimmune diseases and has been reported mainly in Japan [1], with a single case reported in South Korea [2], and a few confirmed cases outside Asia. However, there are no reports of PIG in kidney transplant patients. Here, we report a patient in South Korea diagnosed with PIG after kidney transplantation.
A 47-year-old woman presented to the nephrology department of our hospital to evaluate proteinuria. She had a history of hypertension and gout and was diagnosed with systemic lupus erythematosus (SLE) in 1991 and nephritic syndrome in 1997. The nephritic syndrome was suspected to be lupus nephritis clinically, but was not proven pathologically. In 2007, she underwent living-donor kidney transplantation (her brother was the donor). The patient was group AB/Rh+ and the donor was group A/Rh+. The HLA (define acronym) crossmatch tests, including complement-dependent cytotoxicity, anti-human globulin, and flow cytometry, were negative. After kidney transplantation, she was maintained on triple immunosuppression therapy with cyclosporine, mycophenolate mofetil, and prednisone. Her kidney function was assessed regularly, and the results had been unremarkable for 14 years. However, increasing proteinuria was detected using dipstick tests, although her blood pressure was normal. She was hospitalized when the urine protein level was 3+ on dipstick testing. On admission, a 24-hour urine collection detected 7,242 mg/day of urine protein. The estimated glomerular filtration rate (eGFR) was 59.7 mL/min/1.73 m2, indicative of mild to moderate renal impairment. Subsequently, a renal biopsy of the allograft kidney was done.
On light microscopy, the specimen contained six glomeruli with two showing global sclerosis, mild interstitial fibrosis, and focal tubular atrophy. Most of the non-sclerotic glomeruli were unremarkable histologically, except for one glomerulus showing mild segmental mesangial hypercellularity and non-circumferential hyalin change of the arteriole (Fig. 1). There were traces of C3 on the capillary walls on immunofluorescence staining, while immunohistochemical staining for BK virus and C4d were normal.
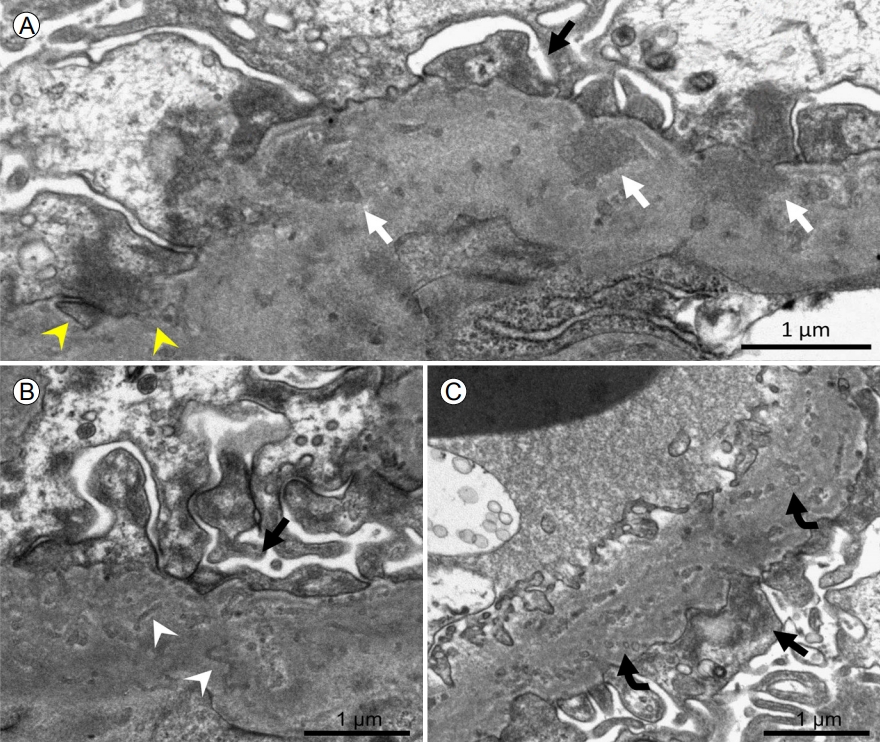
Electron microscopy showed an irregularly thickened GBM (average, 722 nm; 465-752) with sparse subepithelial or intramembranous electron-dense deposition (Fig. 2A) and microtubules (Fig. 2B) or microspherules (Fig. 2C), measuring 35-140 nm in diameter, dispersed throughout the GBM. The podocyte foot processes were diffusely effaced, suggestive of podocyte injury, and the podocyte cytoplasm seemed to invade the GBM (Fig. 2A). The peritubular capillary basement membrane was unremarkable. These findings are compatible with podocyte infolding glomerulopathy (Fig. 2), and are consistent with reports on PIG [3].
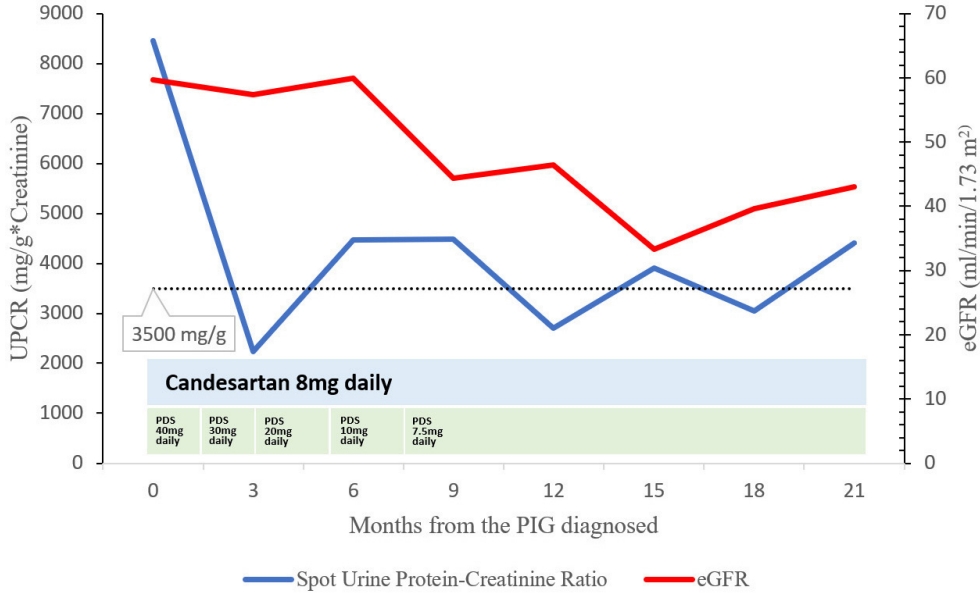
Additional laboratory tests included the following (reference values in parentheses): hemoglobin, 11.1 g/dL; C-reactive protein, 0.1 mg/dL; C3, 104 mg/dL (90-180); C4, 33 mg/dL (10-40); CH50, 59 U/mL (32-58); anti-streptolysin O, 31 IU/mL (0-200); immunoglobulin (Ig) G, 1,228 mg/dL (700-1,600); IgA, 517 mg/dL (70-400); IgM, 188 mg/dL (40-230). Antinuclear antibody was positive at 1:80 titration (speckled pattern). The levels of rheumatoid factor, lupus anticoagulant, anti-cardiolipin, anti-ds-DNA, anti-Smith, and anti-neutrophil cytoplasmic antibodies were normal. Furthermore, phospholipase A2 receptor antibodies and viral markers, including antibodies to hepatitis C virus, hepatitis B surface, and human immunodeficiency virus, were negative. The trough whole-blood cyclosporine level was 241 ng/mL. After diagnosing PIG, the patient was treated with an angiotensin receptor inhibitor (candesartan 8 mg daily) and prednisolone (40 mg daily, then tapered), while on cyclosporine and mycophenolate mofetil for the transplanted kidney. Three months later, the spot urine protein creatinine ratio (UPCR) level had decreased to 2,238 mg/g. However, the UPCR fluctuated and increased to 4,411 mg/g (the nephrotic range is > 3,500 mg/g) 21 weeks after the PIG diagnosis (Fig. 3), while the eGFR decreased from 59.7 to 43 mL/min/1.73 m2 (Fig. 3). The patient was observed to discontinue the drug arbitrarily and this poor compliance appears to have influenced these results.
Most cases of PIG have been reported in northeast Asia, especially Japan [1]. Two cases have been reported in China and a single case in South Korea [2]. PIG has recently been reported outside northeast Asia, including in India, Germany, Argentina, and North America [3]. However, there are no case reports of PIG in kidney transplant patients. Here, we report the first case of PIG in a kidney transplant patient. As in patients with autoimmune diseases such as SLE, PIG could be considered an allograft biopsy finding when glomerular disease is suspected in kidney transplant patients.
PIG usually coexists with autoimmune diseases, including lupus nephritis, Sjögren syndrome, and autoimmune thyroiditis. Cases of PIG without autoimmune disease have also been reported [1]. In the absence of autoimmune disease, PIG is associated with malignancy, hepatitis B virus infection, and membranous nephropathy [3]. Although rare, PIG can appear alone [1]. Our patient was suspected of having lupus nephritis before undergoing kidney transplantation; however, her underlying disease, especially SLE, was well controlled after transplantation, and there was no evidence of lupus nephritis in the renal biopsy pathology or serology examination. Considering these results, although the patient had SLE, lupus nephritis was not observed. There were no kidney transplant complications, including infections or chronic rejection.
In 1985, a ‚Äúnuclear pore complex‚ÄĚ of the GBM, a histopathological finding seen in PIG, was discovered [4]. It was subsequently defined as a new disease entity and was renamed PIG in 2008 [1]. It shows microtubules, microspheres, or both within the GBM, as observed in our case. Although the mechanism of PIG is unknown, podocyte invasion of the GBM could be associated with a membrane attack complex, erroneously formed on the GBM, leading to intra-GBM complement activation [5].
Despite limited data, some mechanisms have been proposed to explain the disease. A case of PIG in a patient with SLE showed several vimentin-positive intra-GBM microstructures and 5b-9 complement complex within microstructures [5]. Since vimentin is a podocyte marker, the pathology suggested that podocytes may be damaged by C5b-9 complex. The pathogenesis of PIG in autoimmune disease may be related to antibody complement-mediated components. Complement activation in autoimmune disease may cause microstructural changes [6,7]. However, this mechanism cannot explain all cases because there have been cases of PIG without autoimmune disease.
Recently reported cases suggest that genetic mutations may be involved in disease development. In a case of PIG with Schimke immuno-osseous dysplasia, novel SMARCAL1 mutations were found and both the patient and his parent with mutations were diagnosed with PIG [8]. Whole exosome sequencing conducted on other patients with PIG found INF2 mutations [3]. These results suggest a relationship between the disease and genetic mutations, and further studies are needed to clarify this. The disease tends to be observed predominantly in Northeast Asians. If differences are observed in the prevalence of these mutations between races, racial differences may be explained.
One case of PIG in multiple myeloma was reported in Japan; the pathogenesis of PIG in multiple myeloma was uncertain, but may have been related to podocyte damage [9]. PIG is also associated with infections and tumors; therefore, several mechanisms could explain the disease. Furthermore, since a patient newly developed focal segmental glomerulosclerosis after PIG was diagnosed [10], it may also affect other diseases. Because PIG has not previously been reported in kidney transplant patients, the relationship between kidney transplantation and PIG is unclear. To understand the clinical course and pathophysiology of PIG, further research is needed.
Acknowledgements
Thank Department of Pathology, Hanyang University Guri Hospital, for technical assistance in this study.
REFERENCES
1. Joh K, Taguchi T, Shigematsu H, et al. Proposal of podocytic infolding glomerulopathy as a new disease entity: a review of 25 cases from nationwide research in Japan. Clin Exp Nephrol 2008;12:421‚Äď431.


2. Kwon KW, Jeong HJ, Lee JH. Podocytic infolding glomerulopathy: a case report. Ultrastruct Pathol 2016;40:374‚Äď377.


3. Feng Y, Wang W, Zou Y, et al. Podocyte infolding glomerulopathy: a case series report and literature review. J Clin Med 2023;12:1088.



4. Dales S, Wallace AC. Nuclear pore complexes deposited in the glomerular basement membrane are associated with autoantibodies in a case of membranous nephritis. J Immunol 1985;134:1588‚Äď1593.


5. Fujigaki Y, Muranaka Y, Sakakima M, et al. Analysis of intra-GBM microstructures in a SLE case with glomerulopathy associated with podocytic infolding. Clin Exp Nephrol 2008;12:432‚Äď439.


6. Nakajima M, Hewitson TD, Mathews DC, Kincaid-Smith P. Localisation of complement components in association with glomerular extracellular particles in various renal diseases. Virchows Arch A Pathol Anat Histopathol 1991;419:267‚Äď272.


7. Hinglais N, Kazatchkine MD, Bhakdi S, et al. Immunohistochemical study of the C5b-9 complex of complement in human kidneys. Kidney Int 1986;30:399‚Äď410.


8. Xiong S, Shuai L, Li X, Dang X, Wu X, He Q. Podocytic infolding in Schimke immuno-osseous dysplasia with novel SMARCAL1 mutations: a case report. BMC Nephrol 2020;21:170.



A glomerulus showed segmental mesangial hypercellularity (arrow) and arteriolar hyalinosis (curved arrow). Periodic acid Schiff, original magnification √ó 200.
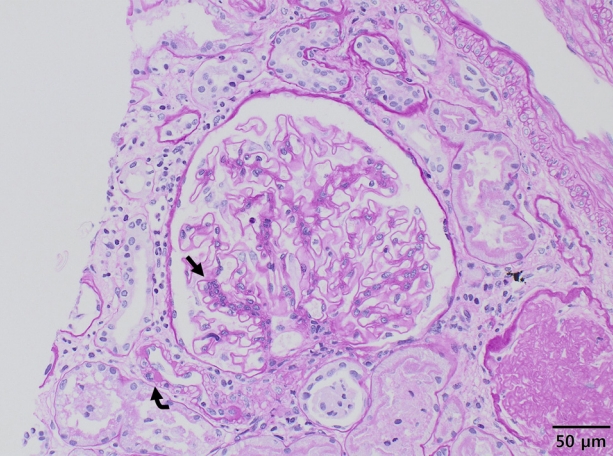
Figure 1.
Electron microscopy showed (A) sparse subepithelial or intramembranous electron-dense deposition (white arrows), invagination of podocyte cytoplasm into the glomerular basement membrane (yellow arrowheads), and (B) microtubules (white arrowheads) or (C) microspherules (curved arrows). Podocyte foot processes are diffusely effaced (black arrow). Transmission electron microscopy, original magnification √ó 10,000.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print




