INTRODUCTION
Recently, most benign esophageal strictures have been treated via endoscopic balloon dilation (EBD) (usually a few sessions) [1]. Esophageal self-expandable stents are widely placed, particularly when treating refractory benign strictures, and stent efficacy and safety have improved over time [2]. Although various stent-related complications have been reported [3], stent flange-induced stricture after stent removal has yet been described. Herein, we report a patient with such a refractory stricture, who was eventually treated using a combination of an oral steroid with repeated EBD.
CASE REPORT
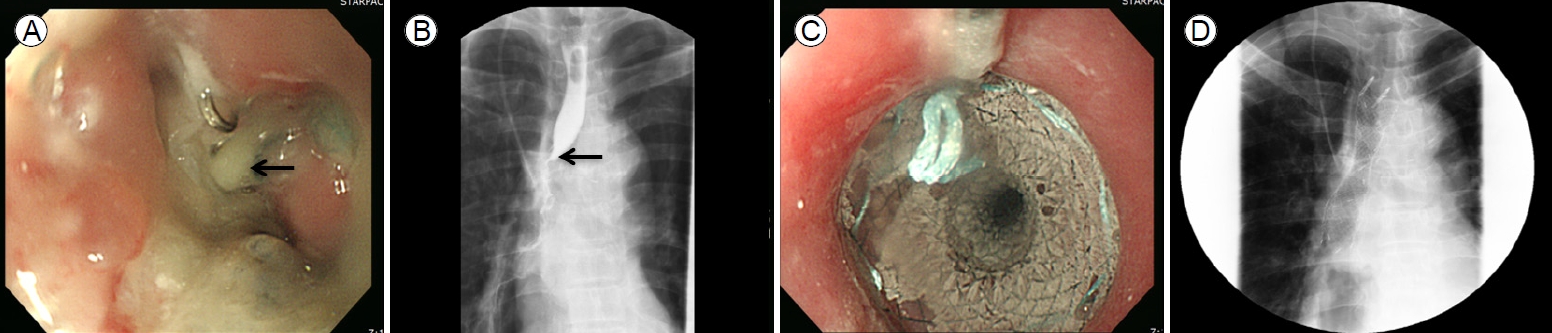
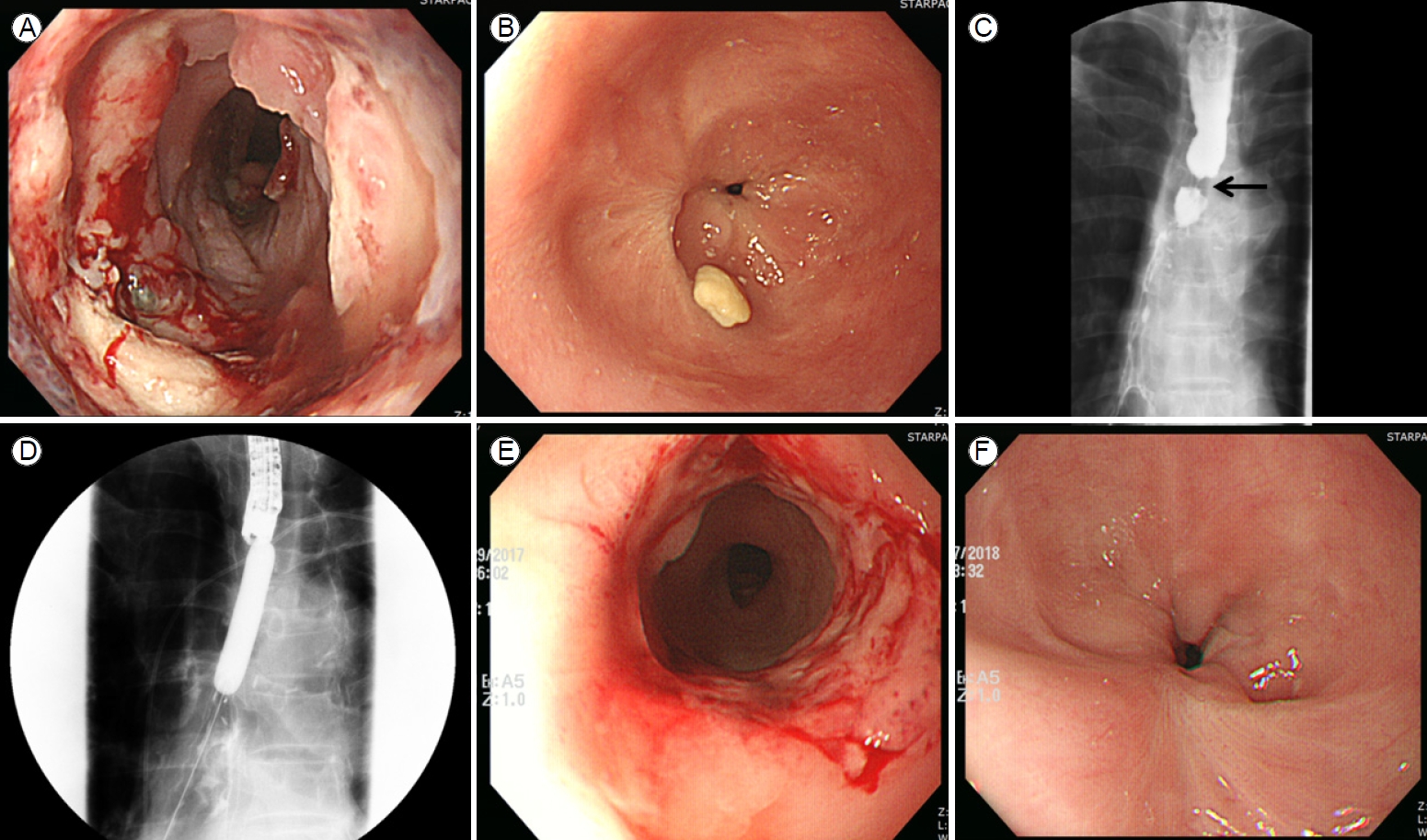
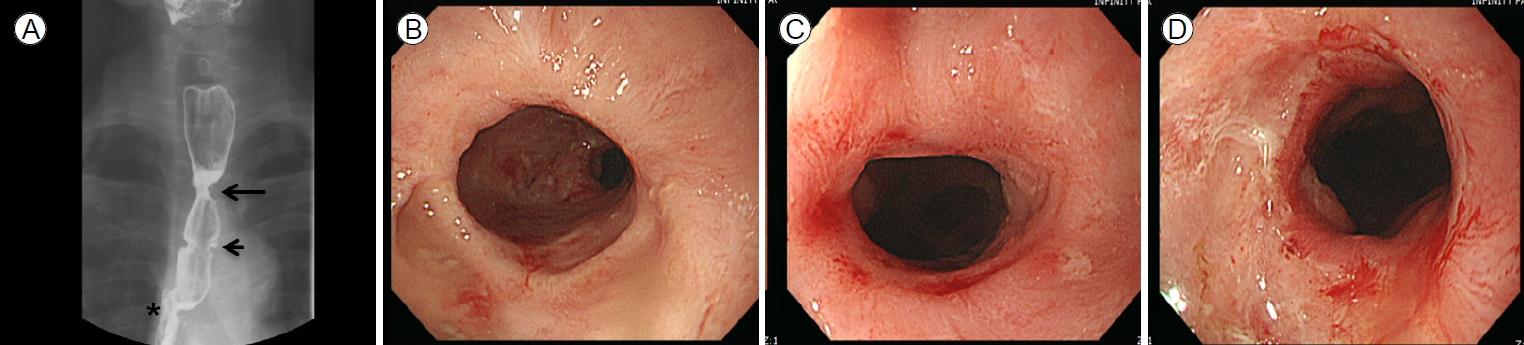
A 70-year-old male complained of dysphagia and was admitted to the department of gastroenterology in Bundang Jesaeng General Hospital. He had a history of anastomotic stricture (Fig. 1A, B) developing 20 days after Ivor-Lewis esophagectomy to treat esophageal cancer in the department of cardiothoracic surgery. He had received a fully covered metallic stent (Niti-S Esophageal Stent; Taewoong, Seoul, South Korea; length 12 cm) (Fig. 1C, D) rather than EBD because of a risk of dehiscence. The stent had remained in place for 2 months and had been removed via esophagogastroduodenoscopy (EGD) 10 days before he was admitted to the department of gastroenterology. At the time of stent removal, endoscopy revealed a circular ulceration with a whitish ischemic change at the proximal flange site (Fig. 2A).
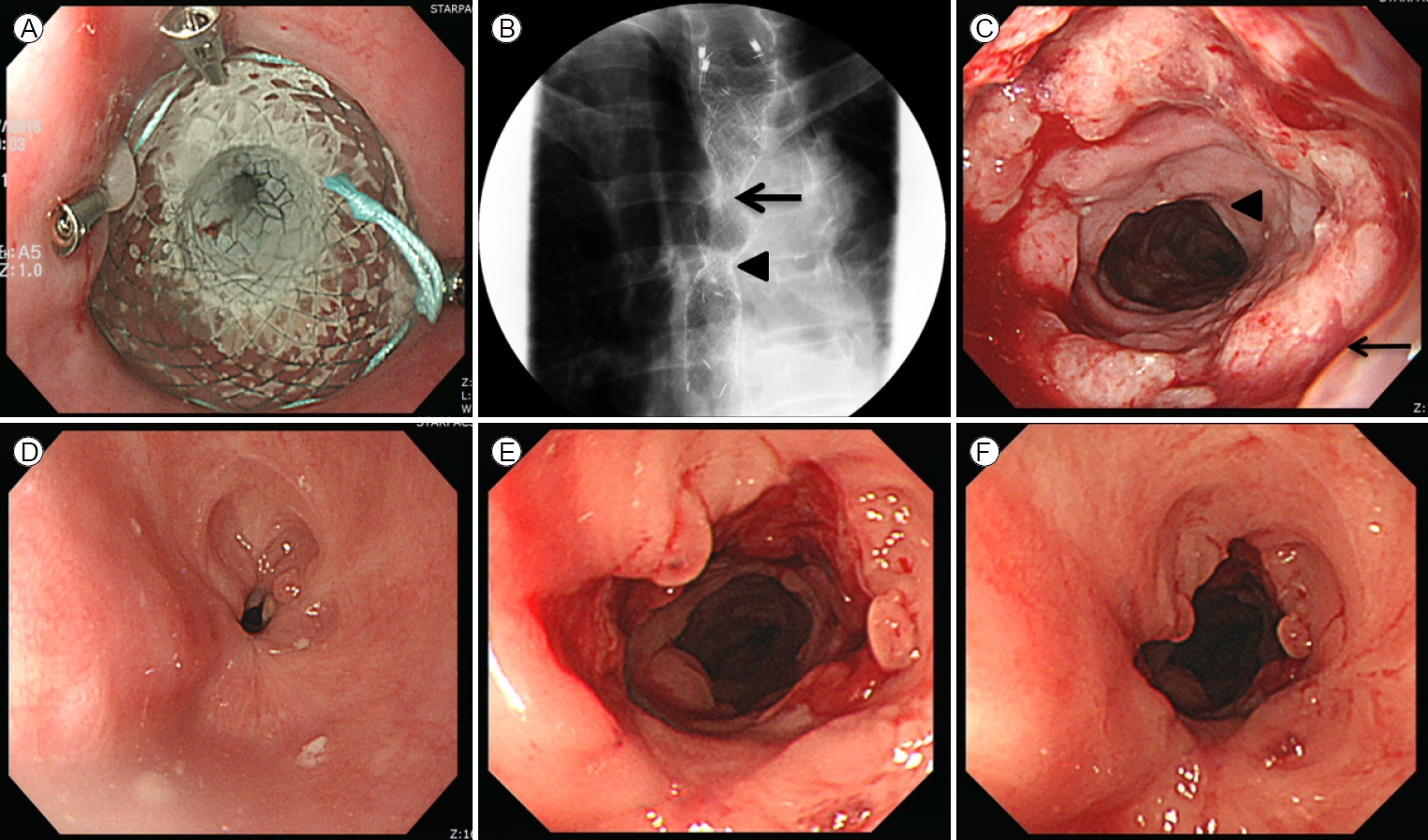
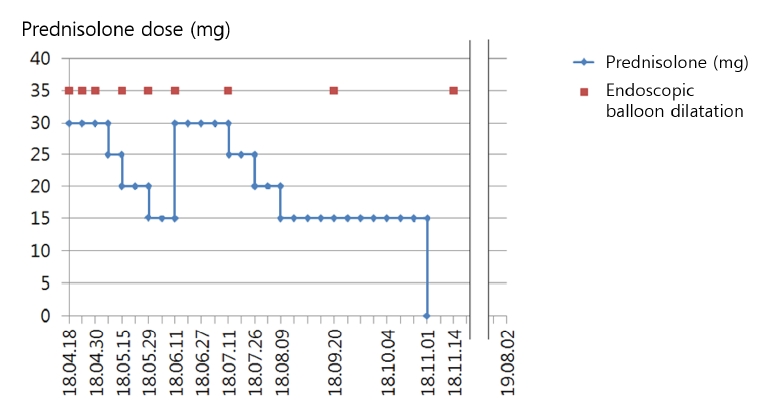
EGD revealed a new stricture at the proximal flange site of the prior stent (Fig. 2B, C). Several sessions of EBD were performed at intervals of 2 weeks (Fig. 2D, E), but the new stricture was refractory to treatment and persistently relapsed (Fig. 2F). Therefore, the patient eventually received a second stent as rescue therapy (Fig. 3A, B), which was scheduled for removal 2 months later. The patient was admitted after 2 months and the second stent was removed via EGD. Endoscopy revealed that although the stricture induced by the first stent and the anastomotic stricture had improved, de novo ulceration was apparent around the proximal flange of the second stent (Fig. 3C). To prevent stricture formation, an oral systemic steroid (prednisolone 30 mg daily) was commenced. A new stricture developed at the proximal site of the second stent (Fig. 3D), but steroid therapy combined with EBD kept the ulcer active (Fig. 3E, F), thus delaying healing and leading to undesirable scarring. After 3 weeks, the steroid was carefully tapered by 5 mg/day over 1 week. However, the stricture deteriorated when the steroid level fell to 15 mg/day. Therefore, the dose was returned to 30 mg/day, maintained for 4 weeks, and then decreased by 5 mg every 2 weeks. When the dose reached 15 mg/day, this was maintained for 10 weeks and then it was completely discontinued (Fig. 4). This reduced the need for EBD sessions; the symptom-free intervals became longer. Dysphagia did not recur within 14 months after stopping the oral steroid and EGD showed that all strictures were tolerable (Fig. 5). Follow-up computed tomography revealed no evidence of cancer recurrence at the anastomosis site.
DISCUSSION
Although esophageal stents can cause various complications, stent flange-induced stricture after stent removal has yet been reported. We initially treated the anastomotic stricture by placing a fully covered metallic stent, but a benign stricture developed at the proximal flange site after stent removal. Although gastric acid reflux, food reflux after operation, external esophageal pressure, and cardiovascular problems may cause strictures [4], it is remarkable that a stent placed to treat a stricture triggered a new stricture.
Anastomotic strictures that develop after esophagectomy are principally attributable to ischemic changes in the anastomosis, leakage, bleeding, and infection of the anastomotic site [5]. Of these various causes, anastomotic ischemia may be closely related to the development of an anastomotic stricture [4]. In our patient, a surgery-induced ischemic change at the anastomosis site was evident via EGD (Fig. 1A), possibly associated with full-thickness inflammation of the esophageal wall. Furthermore, a fully covered metallic stent applies continuous vertical pressure to the luminal wall, which may cause mucosal injury and ulceration [6]. In patients who undergo esophageal endoscopic submucosal dissection (ESD), the risk for a post-ESD stricture increases if the mucosal defect involves > 75% of the luminal circumference or its depth exceeds that of the mucosal lamina propria [7]. In our case, the circular mucosal defect caused by the stent flange included the entire circumference of the esophageal lumen and the depth was greater than that of the mucosal lamina propria (Fig. 2A). Therefore, the abovementioned stricture risks were in play after removal of both the first and second stents.
Recently, oral steroid therapy has been widely used to prevent formation of esophageal ESD-induced strictures [8]. Steroids are anti-inflammatory, inhibit collagen synthesis, and cause collagen fiber breakdown early during healing [9,10]. Ulcer activity is maintained by steroids, thus inhibiting stricture formation. One meta-analysis found that combining a steroid with EBD significantly decreases the number of procedures required to resolve an anastomotic stricture developing after esophagectomy [11]. Based on this, we decided to use an oral steroid to prevent further stricture formation immediately after removal of the second stent.
The recommended oral prednisolone dose is usually 30 mg, tapered after 2 weeks and discontinued after 8 weeks; this prevents post-ESD stricture formation [12]. However, a recent meta-analysis suggested that long-term (Ōēź 12 weeks) oral steroid better reduced stricture formation and the number of EBD sessions than does medium-term (8 weeks) and short-term (3 weeks) steroids [13]. Therefore, we planned to commence with long-term high-dose steroid (prednisolone 30 mg) and reduce it carefully. The steroid suppressed granulation tissue and stricture formation and kept the stent-induced ulcer active. Scar formation was restrained and EBD prevented stricture formation. The dysphagia-free intervals became longer and the number of required EBD sessions decreased. However, when the prednisolone dose was first reduced to 15 mg, the stricture deteriorated. Therefore, we returned the steroid to 30 mg/day for 4 weeks, with careful observation. As the stricture partially improved, the prednisolone dose was slowly tapered (5 mg every 2 weeks) with careful monitoring of dysphagia; the 15 mg/day dose was maintained for 10 weeks, which improved the stricture. Oral prednisolone at less than 15 mg/day can be discontinued without tapering; such a low dose does not prevent stricture (Fig. 4).
In our case, high-dose (30 mg/day) oral prednisolone for over 7 weeks seemed to inhibit flange-induced stricture formation. A moderate dose (25-15 mg) for over 20 weeks reduced stricture recurrence as the stricture of concern improved.
It would have been better to prescribe steroids from the time of development of the first stent flange-induced stricture. Local steroid injection could prevent post-ESD stricture [14]. However, steroid may trigger deep muscle injury when injected into the muscularis propria [8]. Our patient lacked all mucosa and submucosa near the flange-induced ulcer; we decided that an oral steroid was safer than a local steroid injection.
Endoscopic radial mucosal incision can be used to treat benign refractory strictures. Fibrotic tissue is cut away after radial incision of the stricture using an insulated-tip knife [15]. This rapidly relieves dysphagia symptoms. However, re-treatment or additional treatment is often required to maintain long-term luminal patency [16]. In our case, as the strictures were provoked by full-thickness inflammation accompanied by stent flange-induced mucosal damage, radial mucosal incision would not have been more appropriate than EBD. In addition, this procedure is not yet covered by Korean National Health Insurance System.
This is the first report of treatment of a stent flange-induced refractory stricture via a long-term high- to moderate-dose oral steroid combined with repeated EBD (Fig. 6, Table 1). We suggest that a stent flange may be able to induce a refractory stricture that can be managed as we have described.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print






