 |
 |
| Korean J Med > Volume 95(4); 2020 > Article |
|
Abstract
Mycobacterium massiliense (M. massiliense) is a novel nontuberculous mycobacteria (NTM) and an opportunistic pathogen that lives in the water, soil, food, and air. It is a subspecies of the rapidly growing mycobacteria Mycobacterium abscessus. This atypical pathogen has been reported mainly in patients with lung disease or those undergoing cosmetic or surgical procedures. A 62-year-old woman presented with productive otorrhea for 10 months, no history of surgery, and chronic otitis media. M. massiliense was identified from a tissue specimen using real-time polymerase chain reaction for NTM (Biosewoom, Seoul, Korea), and NTM was identified by acid-fast bacilli culture. Successful treatment consisted of clarithromycin for 4 months. No other case of chronic otitis media related to M. massiliense has been reported. This is the first confirmed case of chronic otitis media caused by M. massiliense in a healthy adult in South Korea.
Mycobacterium massiliense (M. massiliense) is a species within the Mycobacterium abscessus complex of three closely related subspecies: M. massiliense, M. bolletii, and M. abscessus [1]. Pulmonary, skin, and soft tissue infections caused by M. massiliense have been reported [2,3]. While otitis media due to nontuberculous mycobacteria (NTM) is common in children, it is rare in adults. Many M. massiliense infections related to iatrogenic procedures have been reported. Here, we firstly report a patient with chronic otitis media due to M. massiliense in a healthy adult in South Korea that was not caused by an iatrogenic procedure.
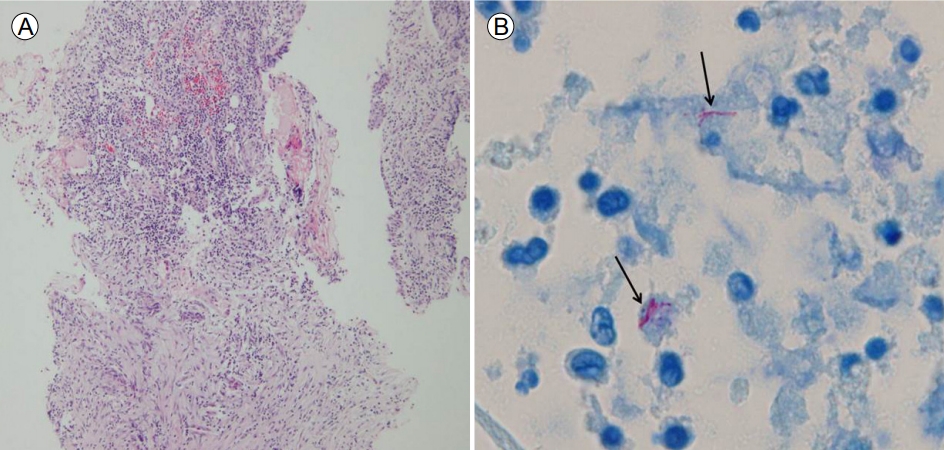
A 62-year-old woman presented with left-sided otalgia with otorrhea for 10 months, despite being prescribed standard antibiotics for chronic otitis media. Her medical history was unremarkable, with no previous medical, surgical, or cosmetic treatment of the ear. On admission, otoscopy showed perforation of the tympanic membrane with serous otorrhea (Fig. 1A). No bacterias were identified from the initial cultures. Contrast magnetic resonance imaging on coronal and axial views showed diffuse enhancement in the tympanic cavity and antrum, air cells, thickening of the mucosal lining of the external ear, and mild stenosis (yellow arrow of Fig. 2). The serum white blood cell counts and C-reactive protein level were normal. We performed acid-fast bacillus (AFB) staining and a molecular method to detect tuberculosis (TB) and NTM from an otorrhea specimen. AFB staining was positive, but the polymerase chain reaction (PCR) and culture for TB were negative. The PCR and culture for NTM were both positive. Chest computed tomography revealed no evidence of pulmonary TB. The pure-tone audiogram revealed left mixed hearing loss with average bone conduction of 31 dB HL and air conduction of 53 dB HL. A second culture from surgically excised tissue in the tympanic cavity was positive for M. massiliense. After the second culture, the patient was treated with clarithromycin 1,000 mg/day, amikacin 15 mg/kg/day, and cefoxitin 12 g/day. The otorrhea improved within 2 weeks. Ultimately, we diagnosed chronic otitis media caused by M. massiliense. Antibiotic susceptibility tests showed resistance to amikacin (intermediate [I]), cefoxitin (I), ciprofloxacin (resistant [R]), doxycycline (R), imipenem (I), rifampin (R), tobramycin (R), ethambutol (R), and linezolid (R), but not to clarithromycin (susceptible [S]) or trimethoprim/sulfamethoxazole (S). Histopathologically, there was inflammatory and granulation tissue but no caseous necrosis (H&E staining Г—100) (Fig. 3A) and AFB staining showed rod-shaped mycobacteria (H&E staining Г—1,000) (Fig. 3B). Our patient was given clarithromycin for 4 months and remained well, with no evidence of recurrence at 12 months. Follow-up otoscopy revealed improvement in the tympanic cavity and external ear (Fig. 1B).
Middle ear infection is a common community disease and the usual pathogens are bacteria. NTM is occasionally associated with middle ear infection because NTM has low pathogenicity and causes localized infections, mainly in children. The most common forms of NTM disease are lung disease, lymphadenitis, skin/soft tissue infection, keratitis, arthritis, meningitis, and endocarditis [4]. Most middle ear NTM infections are caused by the M. fortuitum (M. fortuitum, M. chelonae, and M. abscessus; 67%) and M. avium (27%) complexes [5]. To our knowledge, only 10 cases of chronic otitis media caused by M. abscesses have been reported, two of which were in adults [6-8]. These involved otitis media and otomastoiditis due to M. massiliense in immunocompromised adults in Taiwan [5]. In our patient, AFB staining, PCR, and NTM culture of the ear discharge were positive. This is a very rare case of chronic otitis media caused by M. massiliense in a healthy adult.
A Brazilian study reported 74 post-surgical infections caused by M. massiliense [9]. There have also been reports of skin and soft tissue infections [4,10], post-cesarean section wound infections [11], a post-procedural outbreak associated with ultrasound transmission gel [12], otitis media [5], and endocarditis [13]. Many M. massiliense infections related to iatrogenic procedures or the use of medical appliances have been reported. However, our patient had not undergone surgery or used medical appliances. We identified the pathogen by surgical excision biopsy. A previous study found that the main route of NTM entry is via tympanostomy tube insertion [5]. Other routes include the eustachian tube, via a hematogenous route, or direct spread. M. massiliense was also found to penetrate the skin barrier and invade the blood stream despite the routine use of an adhesive dressing to cover a wound. One study reported that NTM is highly resistant to disinfectants [14], and two studies found that NTM was more transmissible and virulent than other M. abscessus complex pathogens [14,15]. We postulate that our patient was infected during an otologic examination or via other environmental factors.
The treatment of M. massiliense infections is difficult because it is resistant to standard antibiotic and anti-tuberculous medications. However, surgery in combination with long-term antimycobacterial therapy has been reported to have good results. Compared with M. abscessus, M. massiliense responds well to clarithromycin-based treatment [1]. Concerning the antibiotic susceptibility of M. massiliense, two studies reported that M. massiliense is susceptible to clarithromycin and amikacin but resistant to other antibiotics [1,4]. Inducible clarithromycin resistance has been reported in M. abscessus but not in M. massiliense isolates. The treatment response rates to antibiotic therapies including clarithromycin in NTM-related lung disease are considerably higher in patients infected with M. massiliense compared with M. abscessus [1]. One patient received clarithromycin monotherapy for 6 months for M. massiliense infection of the skin and soft tissue of the right lower leg, which was not caused by a medical appliance [4]. In our patient, the M. massiliense was susceptible only to clarithromycin, and she was treated successfully with clarithromycin monotherapy, without surgical excision of the infected tissue. We recommend NTM testing for patients presenting with chronic infection or with a standard antibiotic-resistant clinical course, even in adults and immunocompromised patients.
In conclusion, we report a rare case of chronic otitis media caused by M. massiliense in a healthy adult. This is the first reported case in South Korea. While rare, M. massiliense should be considered as a possible cause of chronic otitis media in adults. For an accurate diagnosis of M. massiliense, mycobacterial culture, antibiotic susceptibility testing, and molecular methods are necessary.
Acknowledgements
The study was approved by the Institutional Review Board (IRB) at the Jeju National University Hospital (IRB No. 2019-01-002).
REFERENCES
1. Koh WJ, Jeon K, Lee NY, et al. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med 2011;183:405вҖ“410.


2. Cheng A, Liu YC, Chen ML, et al. Extrapulmonary infections caused by a dominant strain of Mycobacterium massiliense (Mycobacterium abscessus subspecies bolletii). Clin Microbiol Infect 2013;19:E473вҖ“E482.

3. Duarte RS, LourenГ§o MC, Fonseca Lde S, et al. Epidemic of postsurgical infections caused by Mycobacterium massiliense. J Clin Microbiol 2009;47:2149вҖ“2155.

4. Kim TH, Yoon JH, Jin SJ, et al. A case of skin and soft tissue infection by Mycobacterium massiliense. Korean J Med 2014;87:510вҖ“513.

5. Lee MR, Tsai HY, Cheng A, et al. Otitis media and otomastoiditis caused by Mycobacterium massiliense (Mycobacterium abscessus subsp. bolletii). J Clin Microbiol 2012;50:3754вҖ“3756.



6. Kim HY, Kook Y, Yun YJ, et al. Proportions of Mycobacterium massiliense and Mycobacterium bolletii strains among Korean Mycobacterium chelonae-Mycobacterium abscessus group isolates. J Clin Microbiol 2008;46:3384вҖ“3390.



7. Sugimoto H, Ito M, Hatano M, Nakanishi Y, Maruyama Y, Yoshizaki T. A case of chronic otitis media caused by Mycobacterium abscessus. Auris Nasus Larynx 2010;37:636вҖ“639.


8. Tang IP, Singh S, Rajagopalan R. Bilateral nontuberculous mycobacterial middle ear infection: a rare case. Ear Nose Throat J 2014;93:390вҖ“394.

9. Redaelli de Zinis LO, Tironi A, Nassif N, Ghizzardi D. Temporal bone infection caused by atypical mycobacterium: case report and review of the literature. Otol Neurotol 2003;24:843вҖ“849.


10. Kim HY, Yun YJ, Park CG, et al. Outbreak of Mycobacterium massiliense infection associated with intramuscular injections. J Clin Microbiol 2007;45:3127вҖ“3130.



11. Wu TS, Yang CH, Brown-Elliott BA, et al. Postcesarean section wound infection caused by Mycobacterium massiliense. J Micrbiol Immunol Infect 2016;49:955вҖ“961.

12. Cheng A, Sheng WH, Huang YC, et al. Prolonged postprocedural outbreak of Mycobacterium massiliense infections associated with ultrasound transmission gel. Clin Microbiol Infect 2016;22:382. e1-e382.e11


13. Huth RG, Douglass E, Mondy K, Vasireddy S, Wallace RJ Jr. Treatment of Mycobacterium abscessus subsp. massiliense tricuspid valve endocarditis. Emerg Infect Dis 2015;21:535вҖ“537.



(A) At otoscopy on admission, the tympanic membrane is completely perforated, with serous otorrhea. (B) The external otitis improved after antibiotic treatment for 4 months.
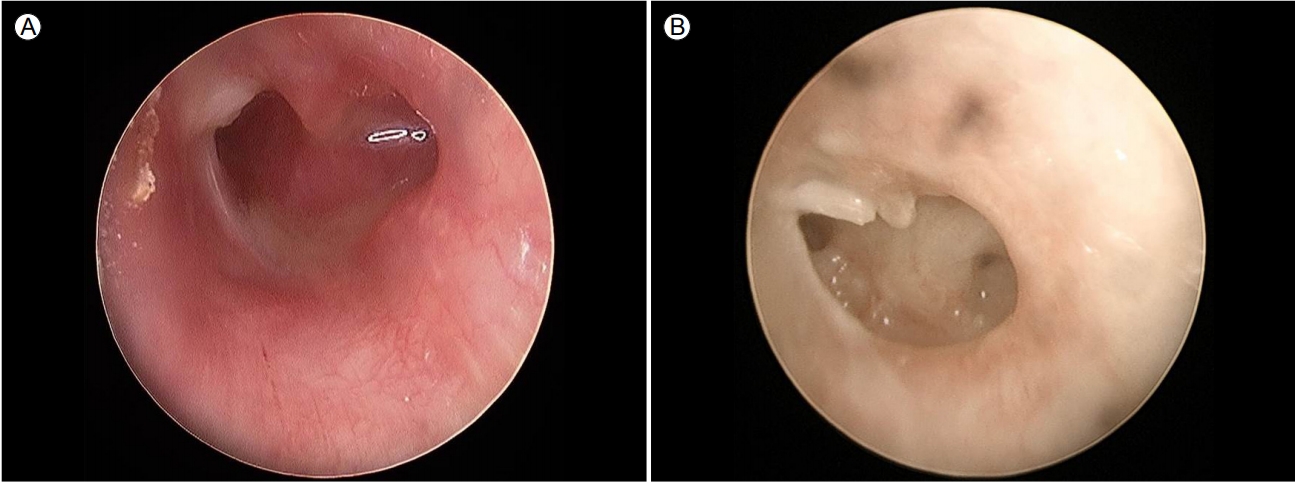
FigureВ 1.
-
METRICS

-
- 1 Crossref
- 0 Scopus
- 3,202 View
- 83 Download




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



