INTRODUCTION
Hemoperitoneum is an infrequent but well-known complication of peritoneal dialysis (PD). It may be caused by trauma, catheter-related malfunction, rupture or perforation of intra-abdominal organs, blood coagulation disorders (e.g., thrombocytopenia), medication (e.g., anticoagulant therapy), malignancy, or actions of the peritoneal muscles [1,2]. Among these various causes, splenic rupture, especially atraumatic splenic rupture (ASR), is a rare occurrence; however, it is associated with a high morbidity and mortality. Here, we report a case of ASR in a patient undergoing peritoneal dialysis (PD) who did not have a history of recent trauma or other co-morbid conditions.
CASE REPORT
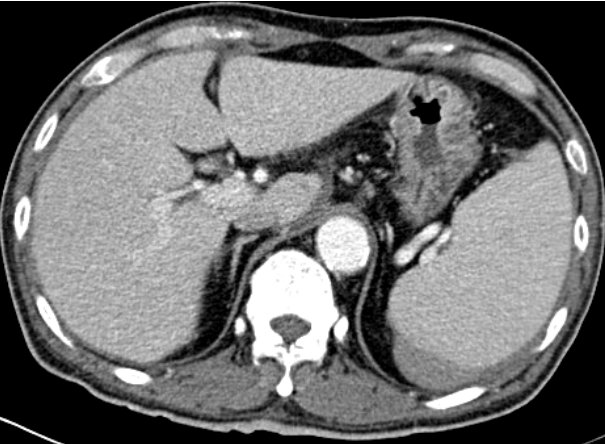
A 66-year-old male undergoing PD presented to emergency room of Veterans Healthcare System Medical Center with diffuse abdominal pain and a pinkish dialysate. He had started PD 11 years prior because of chronic kidney failure of unknown etiology. He had hypertension and a history of parathyroidectomy due to secondary hyperparathyroidism. He had no recent trauma or procedure such as esophago-gastroscopy or colonoscopy, nor was he taking any antiplatelet or anticoagulant agents. While in the emergency department, he was alert and orientated. His vital signs were as follows: body temperature 36.5┬░C, blood pressure 113/76 mmHg, heart rate 110 beats per minute, respiratory rate 20 times per minute, and oxygen saturation of 99%. Left upper quadrant abdominal tenderness without rebound tenderness was noted. The initial laboratory data revealed the following: hemoglobin 10.2 g/dL, hematocrit 33.2%, white blood cells 6,610/mL, platelets 107,000/mL, serum sodium 136 mEq/L, potassium 4.3 mEq/L, chloride 93 mEq/L, blood urea nitrogen 93.5 mg/dL, and creatinine 15.7 mg/dL. His liver enzymes and coagulation studies were within normal ranges. A peripheral blood smear showed no atypical findings. The other routine laboratory findings were unremarkable. No occult blood was detected in fecal sample. Peritoneal fluid analysis showed a white blood cell count of 12/╬╝L without neutrophil predominance (29%). The results of peritoneal fluid and blood cultures were negative. A contrast- enhanced abdominal computed tomography (CT) scan (Fig. 1) showed splenomegaly and a subcapsular hematoma on the posterior aspect of the peri-splenic space. A PD catheter was placed in the rectovesical pouch without complication. Laboratory tests performed to identify the cause of the splenomegaly included hepatitis B, C antibody test, and peripheral blood smear, but none of the results were abnormal. The patient denied history of alcoholism.
Because the patient had stable vital signs and the color of the dialysate became clear, at the time of the first CT scan he was managed conservatively. However, despite trans fusion of two units of packed red blood cells, his hemoglobin level decreased to 8.9 g/dL. A second CT (Fig. 2) scan showed an enlargement of the perisplenic hematoma and a high-density fluid collection, but there was no evidence of active bleeding. To rule out that the PD catheter was the irritant, we decided to temporarily change the patientŌĆÖs mode of renal replacement therapy to hemodialysis. Nonetheless, his anemia progressed, with repeated findings of blood-stained dialysate.
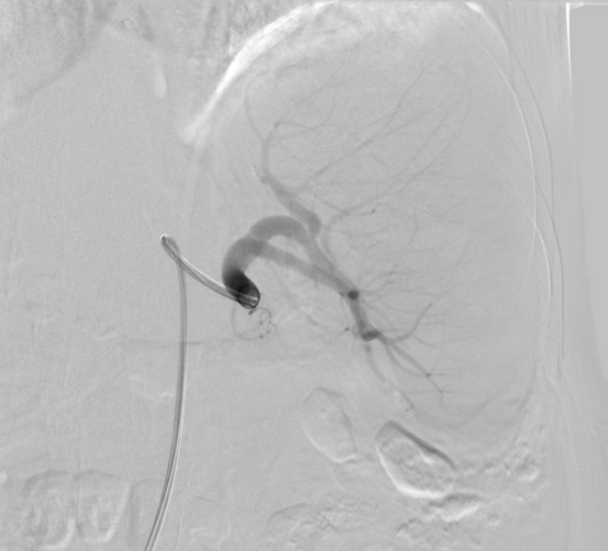
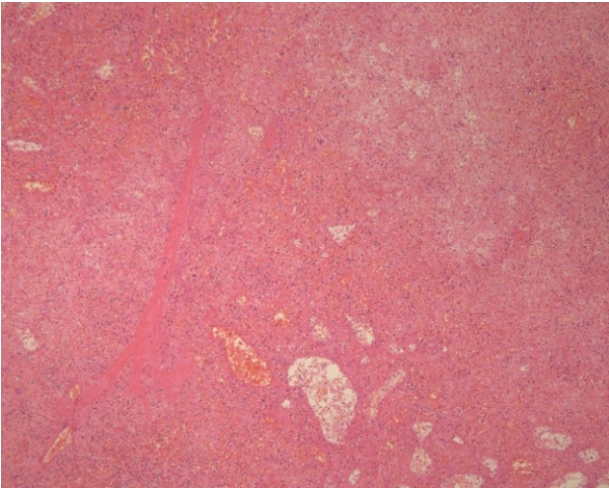
Arteriography (Fig. 3) of the celiac tripod was performed but did not reveal the source of bleeding. However, the splenic artery was embolized preventively. The following day, open splenectomy was performed and the peritoneal catheter was removed. After surgery, the patientŌĆÖs vital signs and anemia stabilized. A third CT scan no longer showed abnormal fluid collection or hemorrhage. The histology of the spleen and splenic vessels was unremarkable (Fig. 4).
DISCUSSION
None of the usual causes of ASR (Table 1), which include infection, inflammation, neoplastic disorders, and medication history [3,4], was identified in our patient. Instead, the cause was determined to be related to PD. Since 1986, nine cases of ASR in PD patents have been reported. Two were treated conservatively and the remainder by splenectomy. Although conservative therapy is an option for patients whose condition is hemodynamically stable [4], total splenectomy is otherwise needed and also allows diagnosis of the underlying condition [5]. However, splenic artery embolization to preserve splenic volume and avoid splenectomy as well as its potential complications is also recommended [6,7].
Hemoperitoneum in PD patients is most commonly due to infection. Our patient had several previous episodes of hemoperitoneum, mostly due to a misplaced catheter and peritonitis, neither of which had been adequately treated. The early diagnosis and treatment of ASR are important as it can be fatal. Abdominal ultrasonography or CT are the most reliable diagnostic methods. On ultrasound, the signs of splenic rupture are enlargement, displacement, double contour, irregularity of the spleen, and intraperitoneal fluid [8]. The CT signs include intracapsular, perirenal, and intraperitoneal fluid [9,10].
In summary, although ASR is a very rare condition, it can be fatal. An early diagnosis is therefore essential. Clinicians should be aware of the potential development of ASR in their PD patients and educate them about its signs and symptoms, as an early intervention may be life-saving.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print






