 |
 |
| Korean J Med > Volume 94(3); 2019 > Article |
|
Abstract
The incidence of rectal neuroendocrine tumors (NETs) has increased worldwide, including in Korea. Rectal NETs are usually single lesions, but synchronous multiple lesions are reported in 2-4.5% of patients. Small rectal NETs (≤ 10 mm) are usually confined to the submucosal layer and rarely give rise to lymph node or distant metastases. Here we describe the case of a 54-year-old woman referred to National Cancer Center for the management of two rectal subepithelial tumors. Because computed tomography revealed a small hepatic nodule suggesting atypical hemangioma rather than metastasis, endoscopic submucosal dissection was performed. However, the size of the nodules increased during follow-up. The pathologic results of a liver biopsy confirmed metastatic NET. This case was unusual in that synchronous small rectal NETs and distant liver metastasis occurred in the absence of any risk factors for metastasis. Thus, patients with rectal NETs should be carefully evaluated, especially for the possibility of metastasis.
The recent increased use of screening colonoscopy, together with advances in endoscopic treatments, have increased the rate of detection of rectal neuroendocrine tumors (NETs). The prevalence of rectal NETs detected by screening colonoscopy is 0.05-0.07% in the United States [1]. Small rectal NETs (≤ 10 mm) are usually confined to the submucosal layer, and only rarely give rise to lymph node and distant metastasis [2]. The overall rate of distant metastasis from rectal NETs is 2-8% [3]. Risk factors for metastasis include tumor size, muscularis layer invasion, histologic grade, and lymphovascular invasion [3,4], whereas multicentricity of the tumor is not regarded as a definite risk factor for distant metastasis of rectal NETs. Here we report an unusual case of synchronous small rectal NETs that had metastasized to the liver in the absence of any risk factors.
A 54-year-old woman referred herself to our hospital for treatment of rectal subepithelial tumors (SETs). She had undergone screening colonoscopy at a local clinic, during which the SETs were found in the rectum. She had undergone total hysterectomy for uterine myoma 3 years earlier but had no other medical problems. Based on the physical examination, she did not appear ill and her abdomen was soft and flat, with no tenderness. Initial colonoscopy showed two yellowish, smooth, elevated lesions, without erosion or ulceration, on the surface of the rectum (Fig. 1). Endoscopic ultrasonography (EUS) identified well-defined, hypoechoic lesions, 6- and 7-mm in size, located 6 and 4 cm from the anal verge, respectively. Both lesions were located in the third sonographic layer and there was no evidence of invasion. Abdominal computed tomography (CT) revealed no mass-like lesion in the large intestine, and no evidence of regional lymph node enlargement. However, a small low-density nodule was detected in segment 7 of the liver (Fig. 2B). A comparison of the images with CT images acquired 6 months earlier at a local clinic (Fig. 2A) showed that the size and shape of the nodule were similar between the two mage sets. Thus, our radiologist suggested that the lesion represented inflammation or an atypical hemangioma, rather than metastasis. The two rectal SETs were resected by endoscopic submucosal dissection. According to the final pathological report, both lesions were well-differentiated, corresponding to a grade 1 (G1) NET in the World Health Organization (WHO) classification of 2010 [5]. One mass (6 cm from the anal verge) was 5 × 4 mm in size, with a depth of 1.8 mm, indicating submucosal involvement; the other (4 cm from the anal verge) was 7 × 6 mm, with a depth of 3 mm, thus also indicating submucosal involvement. The resection margins were free and there was no angiolymphatic invasion (Fig. 3A and 3B). Both NETs stained positive for synaptophysin and CD56, had a Ki-67 proliferative index of < 1%, and were negative for chromogranin and D2-40 staining (Fig. 3C and 3D).
A follow-up sigmoidoscopy performed 4 months later showed no evidence of local tumor recurrence. However, on an abdominal CT scan, the hepatic nodule had slightly increased in size (Fig. 2C). Liver magnetic resonance imaging (MRI), performed to detect metastasis, revealed a nodule of -12 × 9 mm in segment 7 of the liver, with low signal intensity in the hepatobiliary phase (Fig. 2D) and slightly higher signal intensity on the T2-weighted images. A sono-guided biopsy of the hepatic lesion was performed. The pathological report confirmed a metastatic NET, with a Ki-67 proliferative index of < 1% and immunopositivity for synaptophysin and CD56 (Fig. 4). These properties were similar to those of the previously resected rectal NETs.
Further evaluation to check for other distant metastasis was recommended. After being informed of the options for surgical resection of the rectal and liver tumors, the patient refused rectal surgery but underwent hepatic wedge resection. There was no evidence of recurrence at the postoperative 2-year follow-up examination.
NETs occur at various sites characterized by the presence of neuroendocrine cells. They are currently classified and graded according to the WHO classification of 2010 [5]. The incidence and prevalence of rectal NETs has increased due to the use of screening colonoscopy. According to a recent review, in the past 35 years the recorded incidence of rectal NETs in the United States has increased by 800-1,000% [1].
NETs are diagnosed histopathologically but if the endoscopic findings are typical, they can be diagnosed in the absence of a pathology evaluation, as occurs in > 90% of the cases. On endoscopy, NETs appear as yellowish, smooth, elevated lesions with a normal mucosal surface.
Multivariate analyses have identified several factors predicting tumor metastasis, including a tumor size > 14 mm, an increased mitotic rate, and lymphovascular invasion [2,4]. Soga [6] reported metastasis (lymph nodes or distant) rates of 9.7%, 27.6%, and 56.7% for tumors ≤ 10, 10.1-20.0, and ≥ 20.1 mm in diameter, respectively. Another risk factor that predicts metastasis is muscularis layer invasion [4]. Rectal NETs often metastasize to the lymph nodes, but rarely to distant sites. The most common site of distant metastasis is the liver. In the study by Konishi et al. [7], the rate of liver metastasis for tumors ≤ 10, 10.1-20.0, and ≥ 20.0 mm in diameter was 0%, 2%, and 27%, respectively.
Although multicentricity is not considered a risk factor for metastasis from rectal NETs, cases of lymph node metastasis from multiple small rectal NETs have been reported [8-10]. Table 1 presents a summary of all cases of metastasis from multiple rectal NETs < 10 mm reported in the literature. While lesion multiplicity would seem to be a risk factor for metastasis, evidence supporting this conclusion is insufficient and further investigations of a potential association are needed.
The treatment strategy for rectal NETs is based on their size [4]. For rectal NETs < 10 mm in diameter without risk factors, complete local excision, including endoscopic resection, is recommended [4]. For tumors 10-19 mm in diameter, their higher risk of distant metastasis necessitates endoscopic local resection, transanal resection, or radical rectal resection [4,6]. The efficacy of local therapy is a matter of debate but lymph node dissection may be advisable because of the relatively high likelihood of metastasis [4,6]. However, according to recent data, the clinical outcomes for patients with tumors 10-15 mm in size, without metastasis, are similar between endoscopic treatment and surgical resection [2].
In our practice, if the initial evaluation reveals abnormal findings and metastasis cannot be ruled out, then a serial follow-up is conducted. The guidelines of the European Neuroendocrine Tumor Society recommend annual follow-up for G3 NETs < 10 mm and G1-G3 NETs 10-20 mm using EUS, colonoscopy and MRI [3]. However, the most effective schedule for regular follow-up after local resection in patients who are at low risk of metastasis has yet to be determined [3]. In patients with NET multiplicity, angiolymphatic invasion, or a stage G2 tumor, follow-up examinations by colonoscopy (sigmoidoscopy), CT or MRI, or a serological test (chromogranin A), may be appropriate, even for tumors considered to have been treated completely by radical resection.
The case presented herein shows that small synchronous NETs may give rise to distant metastasis even in the absence of risk factors. Thus, in patients with multiple small NETs at the initial evaluation, a more aggressive work-up for lymph-node or distant metastasis, both before and after local resection, should be performed.
REFERENCES
1. Scherübl H. Rectal carcinoids are on the rise: early detection by screening endoscopy. Endoscopy 2009;41:162–165.


2. Park CH, Cheon JH, Kim JO, et al. Criteria for decision making after endoscopic resection of well-differentiated rectal carcinoids with regard to potential lymphatic spread. Endoscopy 2011;43:790–795.


3. Caplin M, Sundin A, Nillson O, et al. ENETS consensus guidelines for the management of patients with digestive neuroendocrine neoplasms: colorectal neuroendocrine neoplasms. Neuroendocrinology 2012;95:88–97.


4. de Mestier L, Brixi H, Gincul R, Ponchon T, Cadiot G. Updating the management of patients with rectal neuroendocrine tumors. Endoscopy 2013;45:1039–1046.


5. Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th. Lyon: IARC Press, 2010.
6. Soga J. Early-stage carcinoids of the gastrointestinal tract: an analysis of 1914 reported cases. Cancer 2005;103:1587–1595.


7. Konishi T, Watanabe T, Kishimoto J, et al. Prognosis and risk factors of metastasis in colorectal carcinoids: results of a nationwide registry over 15 years. Gut 2007;56:863–868.



8. Toh JW, Henderson C, Yabe TE, Ong E, Chapuis P, Bokey L. Management of sub-5 mm rectal carcinoids with lymph node metastases. Gastroenterol Rep 2015;3:350–354.

Colonoscopy findings. (A) A small (6 × 5 mm) yellowish, smooth, elevated subepithelial lesion located 6 cm from the anal verge (black arrow). (B) Another small (7 × 6 mm) subepithelial lesion with the same appearance located 4 cm from the anal verge (black arrow).
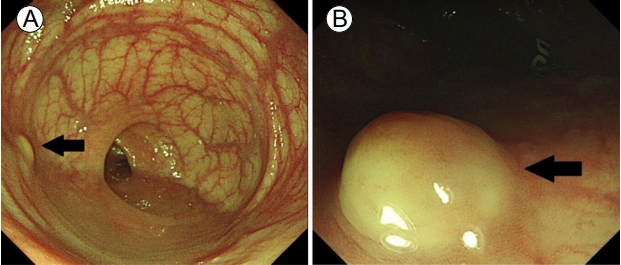
Figure 1.
Abdominal computed tomography (CT) and liver magnetic resonance imaging (MRI) findings. (A) The initial CT scan, taken 6 months before endoscopic submucosal dissection (ESD), shows a small low-attenuating nodule in segment 7 of the liver (black arrow). (B) A CT scan obtained immediately prior to the ESD shows no significant change (black arrow). (C) The CT scan at 4 months after ESD reveals a slight increase in the size of the nodule (black arrow). (D) Liver MRI shows a mass of -1.2 cm with low signal intensity (black arrow) in the hepatobiliary phase.
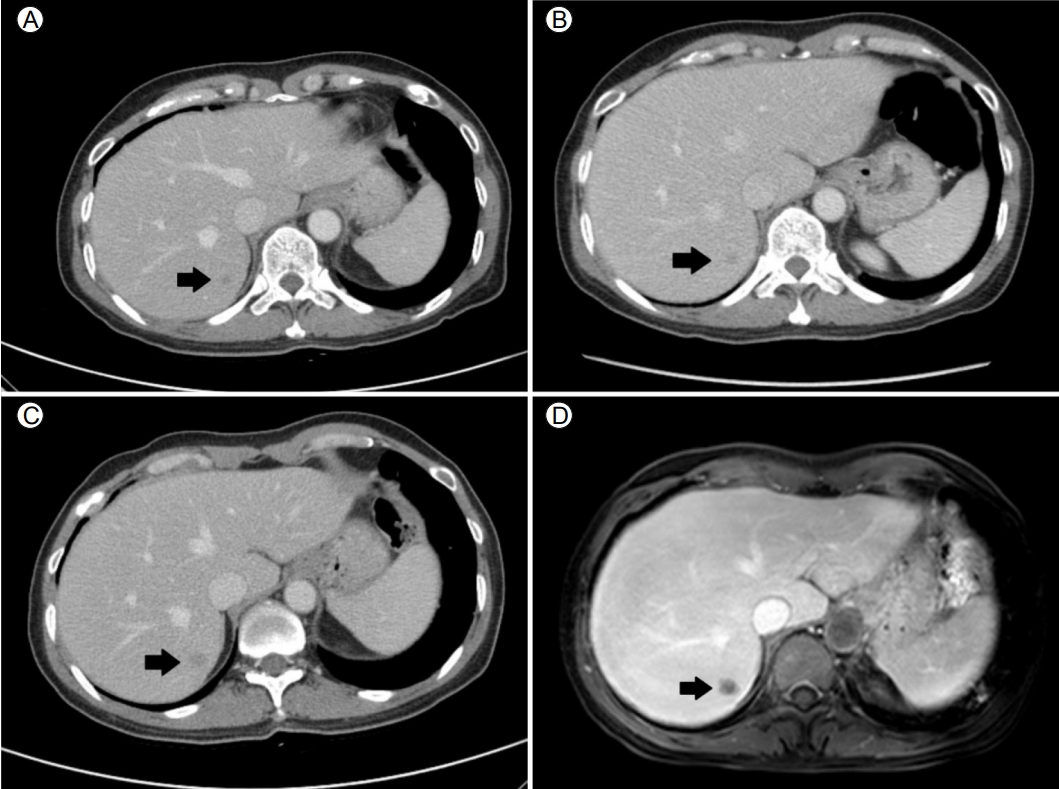
Figure 2.
Pathological findings of rectal neuroendocrine tumors (NETs). The microscopy findings for the subepithelial tumors located 4 cm (H&E stain, ×1) (A) and 6 cm from the anal verge (H&E stain, ×1) (B) include clear resection margins. (C) Monotonous small round tumor cells arranged in an anastomosing ribbon-like pattern (H&E stain, ×200). (D) Diffuse expression of CD56 in the cytoplasm of the tumor cells (immunohistochemical stain, ×200).
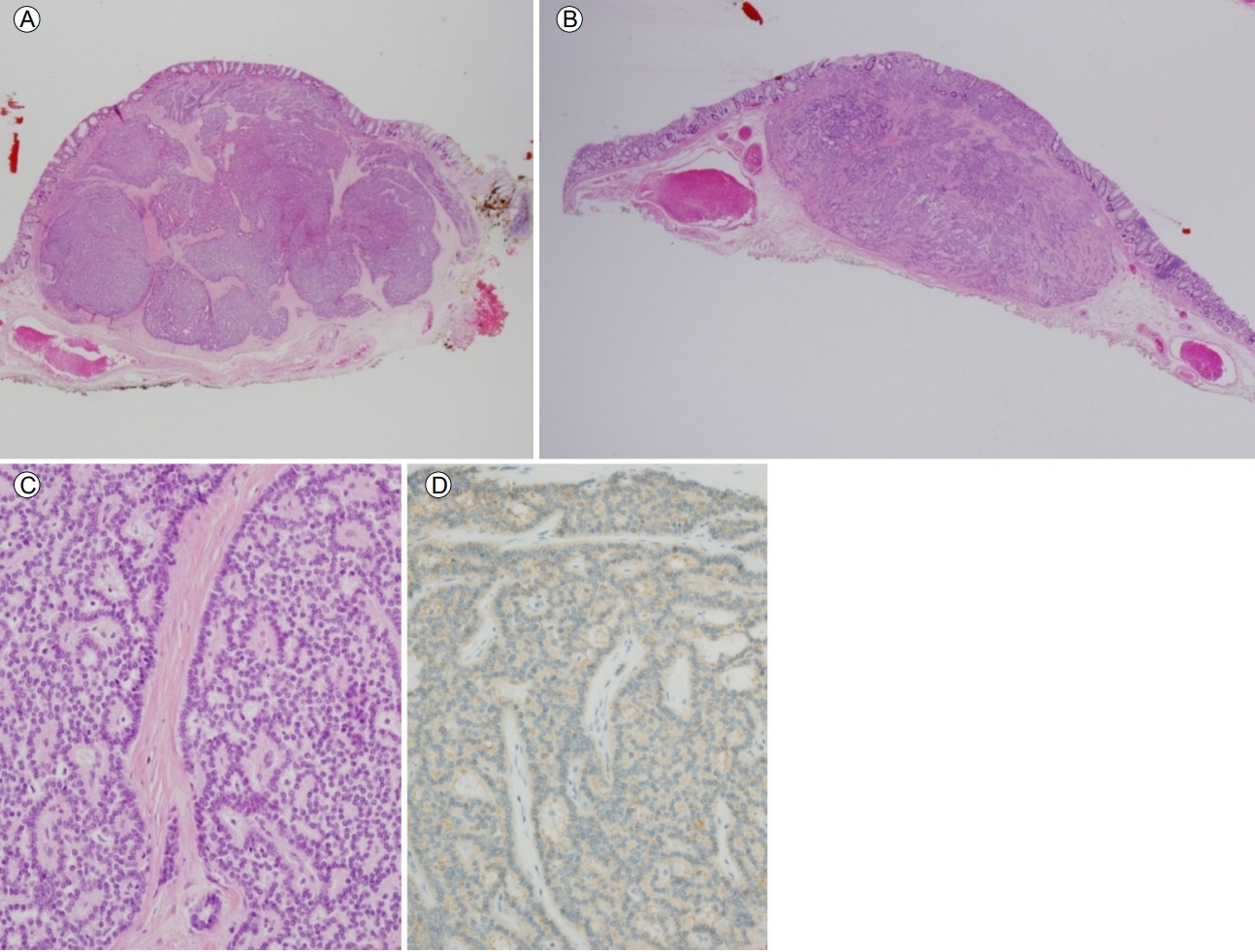
Figure 3.
Pathological findings of a liver biopsy specimen. (A) Monotonous small round tumor cells are arranged in an acinar or solid pattern. The tumor cells are morphologically similar to those of the rectal NET (H&E stain, ×200). (B) The tumor cells are also positive for the cytoplasmic expression of CD56 (immunohistochemical stain, ×200).

Figure 4.
Table 1.
Case reports of multiple rectal neuroendocrine tumors ≤ 10 mm in diameter with metastasis
| Study | Patient sex | Age (years) | Multi-centri city | NET size (mm) | Treatment | WHO grade | Micronests (n) | LN or liver metastasis |
|---|---|---|---|---|---|---|---|---|
| Toh et al. [8] (2015) | Female | 61 | 7 | 0.8-3.5 | uLARa | NET G1 | Yes (5) | Yes |
| Sasou et al. [9] (2012) | Male | 51 | 15 | ≤ 8 | APR | Unknown | Yes (69) | Yes |
| Sasou et al. [9] (2012) | Male | 58 | 3 | ≤ 7 | AR | Unknown | Yes (62) | Yes |
| Kanter and Lechago [10] (1987) | Male | 50 | 17 | < 10 | LAR | NA | NA | Yes |
-
METRICS

-
- 1 Crossref
- 0 Scopus
- 4,293 View
- 123 Download



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



