INTRODUCTION
Pretty first described an intramural hematoma in 1931 and hundreds of cases have since been reported. Nevertheless, it remains a challenging disease due to the poor understanding of its pathophysiology. Spontaneous coronary intimal dissection and rupture with or without atherosclerotic plaque can cause subsequent disruption of the coronary artery and intramural hematoma. Alternatively, spontaneous medial rupture of the vasa vasorum can cause an intramural hematoma without intimal disruption. Either of these events can compress the coronary artery causing angina or acute coronary syndrome. Conventional coronary angiography alone is often insufficient to identify an intramural hematoma without intimal dissection and a visible flap. Intravascular ultrasound (IVUS) and optical coherence tomography (OCT) are helpful modalities, although the optimal treatment options are not well established and case dependent. Here, we present an intramural hematoma in a minimally diseased coronary artery without definite intimal disruption that presented with ST-elevation myocardial infarction in an elderly man who had no risk factors for atherosclerotic disease.
CASE REPORT
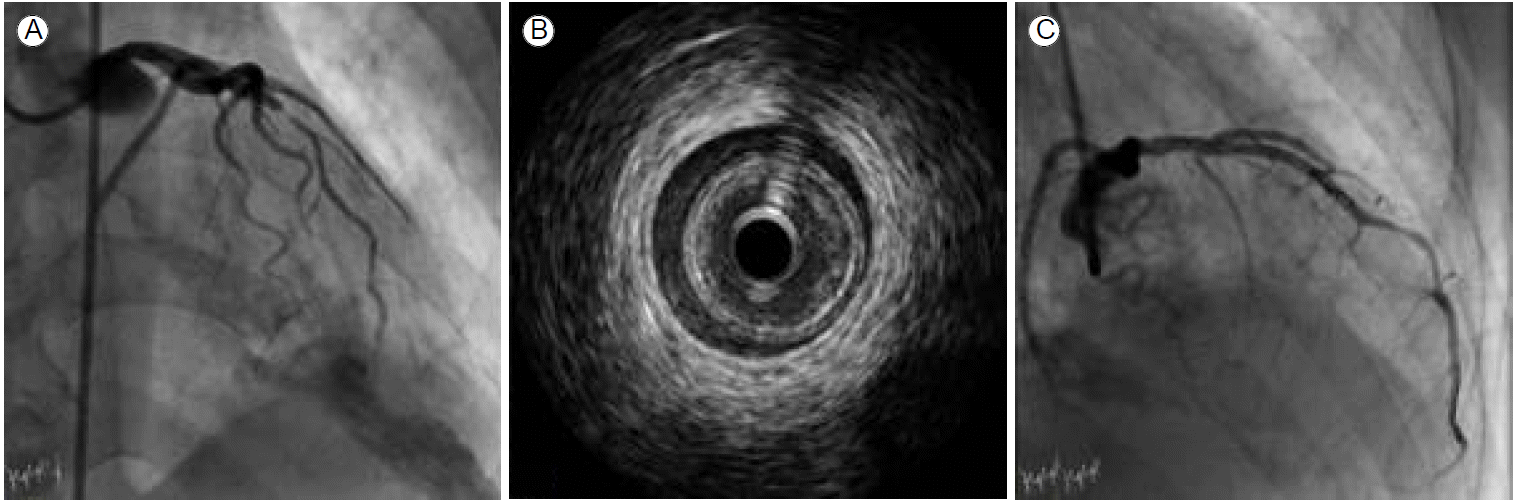
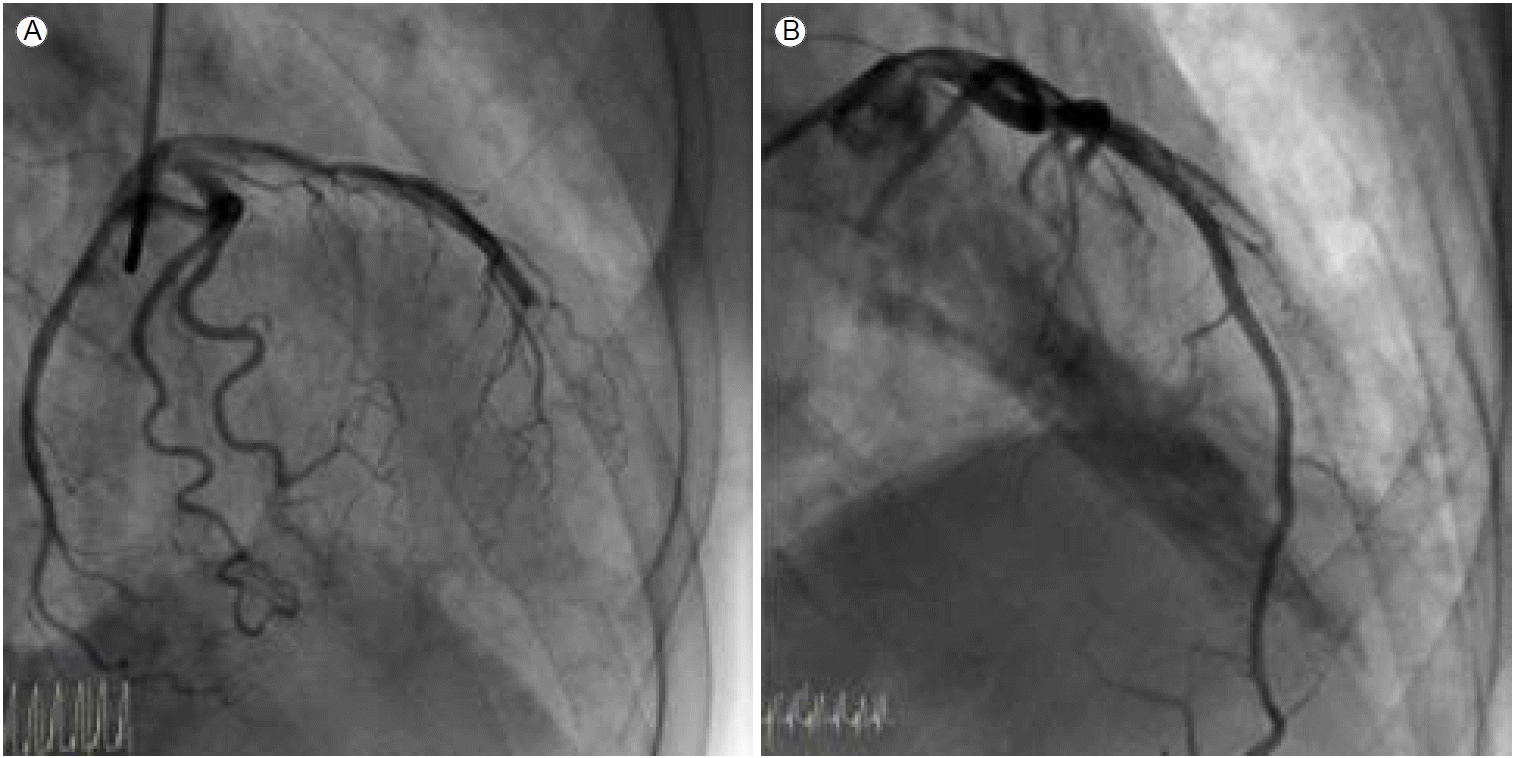
A 67-year-old man with a history of chronic hepatitis C and pneumonectomy for destructive aspergillosis presented with severe ongoing substernal pain that had persisted for more than 1 hour. He had never smoked and had no conventional cardiovascular risk factors such as hypertension, diabetes mellitus, or dyslipidemia. Initial electrocardiography (ECG) revealed ST-segment elevation in precordial leads V1-V4. The patient was given oral loading doses of 300 mg of aspirin and 600 mg of clopidogrel and an intravenous loading dose of 4,000 units of heparin just before percutaneous coronary intervention (PCI). Emergency coronary angiography revealed one culprit lesion in the mid-left anterior descending (LAD) artery, which showed abrupt, step-like, 90% concentric luminal narrowing with thrombolysis in myocardial infarction (TIMI) flow grade II (Fig. 1A). The other vessels were normal. After pre-ballooning with a 2.5 ├Ч 20 mm coronary balloon, we examined the coronary artery using IVUS to determine the appropriate stent size because of a discrepancy in vessel size. The IVUS image showed a long-segmental intramural hematoma and minimal plaque without a definite intimal tear (Fig. 1B). Due to the patientтАЩs severe chest pain and ongoing ischemia in the area ofthe LAD, we implanted a XIENCE Xpedition stent (3.0 ├Ч 33 mm, Abbott Vascular, Abbott Park, IL, USA), with only nominal pressure required to restore the lumen area. After stenting, IVUS revealed a well-trapped intramural hematoma with no further distal progression and a mild plaque at the distal LAD (Fig. 1C). Since the patientтАЩs activated clotting time (ACT) during the procedure was > 1,500 seconds after administering the standard procedural heparin dosage, we waited for another 20-30 minutes for the ACT level to normalize to < 200 seconds. The patient was admitted to the intensive care unit when the final angiogram and ECG were acceptable and he became symptom-free. After 6 hours, the patient complained of severe chest pain with marked ST segment elevation on the inferolateral ECG leads. Subsequent emergency angiography and IVUS revealed total occlusion of the mid LAD artery distal to the previous stent due to progression of the intramural hematoma (Fig. 2A). After ballooning, a 2.75 ├Ч 28 mm stent (XIENCE Xpedition, Abbott Vascular) was inserted, overlapping the previous stent, and good results were achieved (Fig. 2A and 2B). No remnant intramural hematoma was found on the final IVUS examination. After the procedure, the patient was treated with dual anti-platelet therapy and discharged without complications.
DISCUSSION
A coronary intramural hematoma is a rare cause of acute coronary syndrome. Its pathophysiological mechanism is believed to be medial rupture of the vasa vasorum in the arterial wall and the formation of a hematoma, which leads to intimal and coronary artery dissections [1]. Peri- or postpartum middle-aged females with no known atherosclerotic risk factors are primarily affected, probably because of the hormonal changes that occur during menstruation [2]. Hypertension, vasculitis, and medications have been correlated with intramural hematoma, although the relationships have not been proven.
Intramural hematoma is associated with a poor prognosis and usually seen at autopsy after sudden cardiac death [3]. If the patient presents with acute myocardial infarction, distinguishing between intramural hematoma and atherosclerotic plaque rupture is difficult, especially in cases without an intimal tear and spontaneous coronary dissection, unless IVUS or OCT is performed. Moreover, an intramural hematoma is often noticed after stenting complicated by distal propagation of the hematoma [4]. Therefore, the imaging modality is important in deciding treatment options and guiding PCI in this setting, especially if the patient is lacks conventional risk factors [5,6]. There are no guidelines or consensus regarding the optimal treatment strategy for intramural hematoma and spontaneous coronary dissection. PCI is an appropriate option for localized proximal dissections in large vessels associated with ongoing ischemia or TIMI grade 0-1 coronary flow [7]. PCI with stenting can be helpful in recovering distal flow in a violated vessel in patients with an intramural hematoma that is not resolved with medical management. Although spontaneous resolution of an intramural hematoma without intervention has been reported [8], most case studies reported that stenting was necessary to address ongoing ischemia and decreased antegrade flow [4,9,10].
Our case is unique in that the intramural hematoma presented as ST-elevation myocardial infarction in an elderly male who had no known risk factors and was effectively treated with stent trapping. We examined the possible causes of intramural hematoma by conducting specific blood tests for vasculitis because the patient had no conventional risk factors for cardiovascular diseases and intramural hematoma is not common in elderly males. We did not identify any specific findings that would lead to the diagnosis of a specific disease, which supports this unusual phenomenon. In addition, this highlights the importance of IVUS in guiding treatment strategy, as well as in the detection of complications such as distal intramural hematoma propagation.
Clinicians should be mindful of this rare cause of acute myocardial infarction in patients with no risk factors.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print






