INTRODUCTION
Systemic lupus erythematosus (SLE) is an autoimmune disease that can affect a number of organs including skin, joints, kidney, and heart [1]. Cardiac involvement is evident in more than 50% of lupus patients and is a major cause of morbidity and mortality [2]. Cardiac manifestations range from mild symptoms of pericarditis to life-threatening complications such as myocardial infarction, arrhythmias, valvular disease, and heart failure [2].
Sick sinus syndrome is a disorder of the sinoatrial node caused by abnormal sinus impulse formation and/or propagation [3]. Sick sinus syndrome exhibits diverse electrocardiography (ECG) presentations, such as sinus bradycardia, sinus arrest, sinoatrial block, and alternating episodes of bradycardia and tachycardia [3]. Symptoms related to sick sinus syndrome include dizziness, dyspnea, syncope, heart failure, fatigue, and exercise intolerance. Symptomatic sinus bradycardia is rarely reported as a feature of SLE [4,5]. Here, we report a case of symptomatic sinus bradycardia in a young female patient with SLE who was successfully treated with methylprednisolone pulse therapy.
CASE REPORT
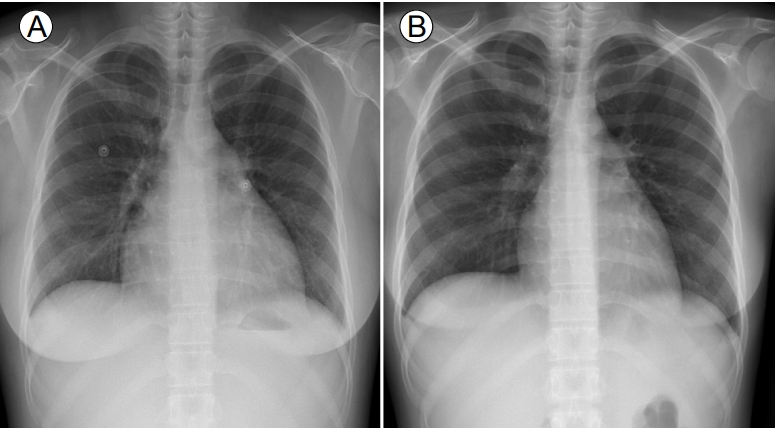
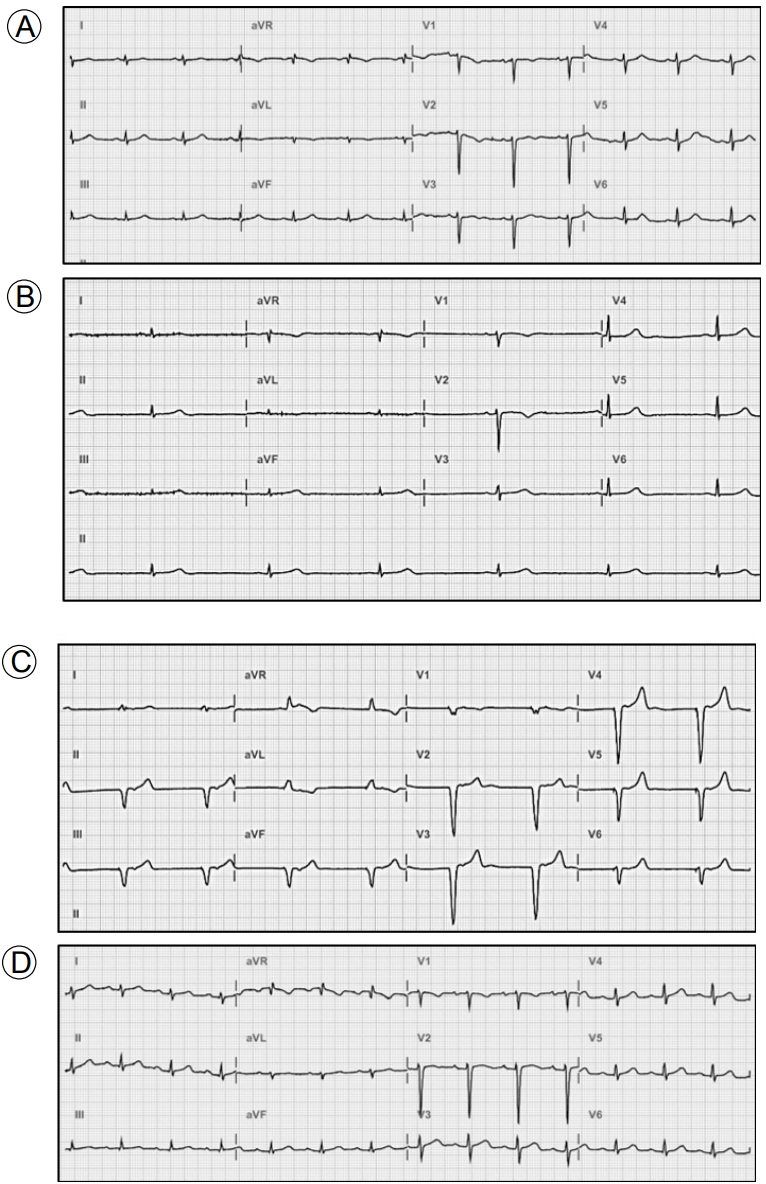
A 24-year-old female with SLE was admitted to Uijeongbu St. MaryŌĆÖs Hospital because of fever, chills, and myalgia. A diagnosis of SLE had been made 5 years prior on the basis of a malar rash, leukopenia, low levels of complement components, and positivity for antinuclear, anti-double stranded DNA, and anti-Smith antibodies. The patient had been prescribed hydroxy-chloroquine but was poorly adherent to therapy. Her temperature was 39.9┬░C, pulse was 105 beats/min, blood pressure was 110/70 mmHg, and respiratory rate was 25 breaths/min. Laboratory tests revealed a white blood cell (WBC) count of 3.700 ├Ś 109/L, a hemoglobin level 8.8 g/dL, a haematocrit of 26.4%, a platelet count of 130 ├Ś 109/L, a urea nitrogen level 24.3 mg/dL, a creatinine level 1.13 mg/dL, a total protein level 6.6 g/dL, an albumin level 2.5 g/dL, an erythrocyte sedimentation rate (ESR) of 33 mm/h (normal: 0ŌĆō15 mm/h), a C-reactive protein (CRP) level 1.3 mg/dL (normal: < 0.3 mg/dL), a procalcitonin level 1.02 ng/mL (normal: < 0.046 ng/mL), a C3 level 16.5 mg/dL (normal: 76ŌĆō139 mg/dL) and a C4 level 2.7 mg/dL (normal: 12ŌĆō37 mg/dL). Urinalysis revealed 20ŌĆō29 WBCs/high power field (HPF), a protein score of 3+, and a protein/creatinine ratio of 9.12. Chest radiography revealed mild cardiomegaly with pulmonary congestion (Fig. 1A). ECG revealed normal sinus rhythm (75 beats/min) (Fig. 2A). Chest and abdominal computed tomography were performed to explore the cause of the fever and revealed small extent of bilateral pleural effusion, pelvic ascites, and periportal edema. The patient was immediately commenced on empirical intravenous ceftriaxone because of presumed pelvic inflammatory disease or urinary tract infection.
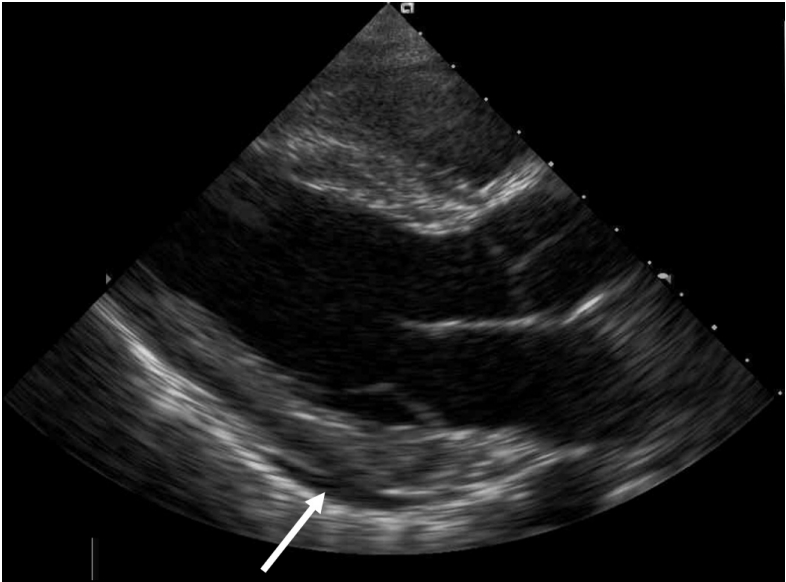
The fever subsided on the third day. However, dyspnea, orthopnea, and dizziness then developed suddenly. The pulse was 36 beats/min, the temperature was 36.6┬░C, and the blood pressure was 127/88 mmHg. Chest radiography revealed no changes. The ECG revealed sinus bradycardia at 35 beats/min with a normal QT interval (Fig. 2B). Laboratory tests yielded the following data: WBCs 5.050 ├Ś 109/L; hemoglobin 8.8 g/dL; platelets 165 ├Ś 109/L; ESR 17 mm/h; CRP level 0.26 mg/dL; creatinine phosphokinase level 19 U/L; creatinine kinase-MB level 0.93 ng/mL (normal: 0.3ŌĆō2.88 ng/mL); troponin T level 0.014 ng/mL (normal: < 0.099 ng/mL); and pro-B type natriuretic peptide level 608.2 pg/mL (normal: 5ŌĆō125 pg/mL). thyroid function tests and serum electrolyte levels were normal. Blood and urine culture results were negative. Transthoracic echocardiography revealed normal regional wall motion, a left ventricular ejection fraction (LVEF) of 57%, normal atrial and ventricular sizes, a normal valvular structure, and a small amount of pericardial effusion (Fig. 3). Holter ECG monitoring revealed sinus bradycardia with a minimum heart rate of 38 beats/min and an average of 46 beats/min. Viral serological tests were negative for hepatitis A, B, and C, herpes simplex virus, influenza, human immunodeficiency virus, cytomegalovirus and Epstein-Barr virus.
We suspected SLE-associated sinus bradycardia and commenced treatment with methylprednisolone after implantation of a temporary transvenous pacemaker (Fig. 2C). She was initially given 125 mg/day methylprednisolone on 2 consecutive days. However, sinus bradycardia persisted on ECG and temporary pacing was required. The dosage was increased to 250 mg/day for 3 days. However, the sinus bradycardia and dizziness persisted. The dosage was then increased to 500 mg/day via pulse infusion for the next 3 days, followed by 60 mg/day of oral prednisolone. After methylprednisolone pulse therapy, the heart rate was maintained at greater than 50 beats/min and the temporary pacing wires were removed (Fig. 2D). Follow-up echocardiography revealed normal ventricular systolic function with an LVEF of 56%, a small amount of pericardial effusion, and left atrial (LA) enlargement (LA volume index 45 mL/m2). 2 days after finishing methylprednisolone pulse therapy, the fever and chills recurred. Her temperature was 38.6┬░C, the pulse 54 beats/min, and the blood pressure 125/80 mmHg. Laboratory tests revealed the following: WBCs 5.650 ├Ś 109/L with neutrophils 74.2%; ESR 17 mm/h; and CRP level 2.57 mg/dL. Urinalysis revealed 20ŌĆō29 WBCs/HPF, a protein score of 1+, and a protein/creatinine ratio of 1.97. Her antibiotic was switched to meropenem to treat a presumed infectious disease and the prednisolone dosage reduced to 10 mg/day to facilitate bacterial killing. After 2 weeks of antibiotic therapy, the fever and chills improved and normal sinus rhythm persisted on the ECG. Follow-up ECG performed 4 weeks after methylprednisolone pulse therapy revealed normal ventricular systolic function and that all chambers were of normal size and lacked pericardial effusion. Cardiomegaly evident on previous chest radiography had disappeared (Fig. 1B). The patient was discharged on 10 mg/day prednisolone. Sinus bradycardia has not recurred during 2 years as an outpatient follow-up on 5 mg/day prednisolone.
DISCUSSION
Cardiac involvement is evident in more than half of all SLE patients and is a primary cause of morbidity and mortality [2]. Pericarditis, myocarditis, coronary artery disease, valvular disease, conduction system abnormalities, and arrhythmias are among the manifestations of cardiac involvement in SLE. Pericarditis is the most common presentation and is reported in 11ŌĆæ54% of SLE patients [2]. Pericarditis, myocarditis, and extensive pericardial effusion can all trigger abnormalities of the secondary conduction system [6]. In this patient, a small amount of pericardial effusion was evident on ECG. However, we found no typical manifestation of acute pericarditis, such as the characteristic chest pain, an ECG abnormality, or a pericardial friction rub; we concluded that the pericardial effusion might be caused by hypoalbuminemia, triggered in turn by proteinuria. Therefore, we excluded pericarditis as the cause of sinus bradycardia. We also excluded myocarditis given the absence of cardiac enzymes in blood and the absence of abnormalities on initial ECG. Most SLE patients with myocarditis exhibit elevated levels of serum markers of cardiac injury and ECG abnormalities including global, regional, or segmental wall motion abnormalities, a reduced EF and an increased chamber size [7]. Sinus bradycardia can also be caused by other conditions such as myocardial ischemia, hypothermia, hypothyroidism, electrolyte imbalance, and/or drugs. In our case, hypothermia, hypothyroidism, and electrolyte imbalance were ruled out by physical examination and laboratory testing. Moreover, the patient had no history of recent medication. Based on the above findings, all other possible causes that could trigger sick sinus syndrome were excluded and we diagnosed isolated sick sinus syndrome caused by SLE.
All chamber sizes were normal on ECG performed at the time of the first episode of sinus bradycardia. However, LA enlargement was observed following implantation of a temporary pacemaker and methylprednisolone infusion therapy. Sinus bradycardia preceded LA enlargement, which was evident on ECG, but the LA size became normalized after removal of the pacemaker. Right ventricular pacing by a pacemaker is known to cause a loss of atrioventricular synchrony and to trigger LA enlargement [8]. The LA enlargement in our case improved after removal of the pacemaker and might therefore have been caused by an adverse reaction to pacemaker implantation.
Symptomatic sinus bradycardia in adults with SLE is rare; no clear association between SLE and sinus bradycardia has yet been demonstrated. The pathogenesis of sinus bradycardia in SLE is believed to involve immune-system-mediated damage to the sinoatrial node or conduction tissue, small vessel vasculitis, and/or antimalarial-induced cardiotoxicity [9,10].
Previously reported cases of symptomatic sinus bradycardia in SLE patients improved after methylprednisolone pulse infusion [4,5]. In our case, we prescribed an initial dose of 125 mg/day rather than pulse infusion because of the concern of an infectious disease; we also referred her for implantation of a temporary pacemaker at that time. However, dizziness caused by sinus bradycardia persisted. To achieve a normal sinus rhythm, the methylprednisolone dose had to be increased to 500 mg/day. After 9 days of methylprednisolone pulse infusion and temporary pacemaker implantation, the sinus bradycardia resolved and the temporary pacemaker was successfully removed. Previous reports have indicated that the responses to methylprednisolone pulse therapy are not always good. Tselios et al. [10] reported that severe brady-arrhythmia, such as a complete atrioventricular block or a sinus pause, required implantation of a permanent pacemaker in 1.32% of SLE patients, and prolonged antimalarial treatment may be associated with severe brady-arrhythmia in such patients. In conclusion, we describe a case of symptomatic sinus bradycardia requiring temporary cardiac pacing in a SLE patient who was successfully treated by methylprednisolone pulse therapy.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print






