INTRODUCTION
Mixed connective tissue disease (MCTD) is an overlap syndrome characterized by the presence of clinical features typical of systemic sclerosis (SSc), systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), polymyositis (PM), and dermatomyositis (DM); MCTD is generally associated with a high titer of U1-ribonucleoprotein (RNP) [1]. The relationship between DM and malignancy is well-recognized [2]; patients with RA, SLE, and SSc are at increased risk of malignancy compared to the general population. Specifically, RA patients are at increased risk of lymphoma, and some reports support the notion that SSc is strongly associated with lung cancer. However, an association between MCTD and malignancy, especially papillary thyroid cancer (PTC), has yet to be established.
Primary biliary cirrhosis (PBC) is an autoimmune hepatic disease characterized by the destruction of intrahepatic bile ducts. This causes fibrosis, eventually leading to liver cirrhosis and high levels of anti-mitochondrial antibody. PBC can involve multiple systems, including lung (interstitial lung disease, ILD), heart (pulmonary artery hypertension, PAH), and kidney (nephritis). In addition, PBC has been reported to be associated with other connective tissue diseases (CTDs) and autoimmune disorders, including Sj├ČgrenŌĆÖs syndrome (SS), SSc, and SLE [3]. However, few reports on the associations between PBC and MCTD have appeared [4]. Herein, we describe co-existing MCTD and PTC in a patient with pre-existing PBC.
CASE REPORT
A 40-year-old female was referred to the hospital complaining of muscle weakness of 8 months in duration, arthralgia and mild exertional dyspnea. In particular, she reported that polyarthralgia had developed in both proximal interphalangeal and ankle joints. On admission, her height was 159.8 cm, weight was 51 kg, and body mass index was 20.2 kg/m2. Her body temperature was 36.8┬░C, her pulse rate was 60 beats/min, blood pressure was 140/70 mmHg, and respiration rate was 24 breaths/min. On physical examination, all proximal interphalangeal joints of both hands and right ankle joint were swollen and tender. The power of both proximal upper limbs and quadriceps muscles was grade 3 on manual muscle testing. RaynaudŌĆÖs phenomenon (RP) was accompanied by telangiectasia and sclerosis of the bilateral fingertips.
PBC had been diagnosed 5 years prior, for which the patient was treated with ursodeoxycholic acid. At the time of the diagnosis, alkaline phosphatase concentration was 981 IU/L (reference value: 35-104) and anti-mitochondrial antibody was positive. On histological examination, a liver biopsy revealed portal inflammation with periportal activity and fibrosis, consistent with clinical findings of PBC (stage 2) (Fig. 1).
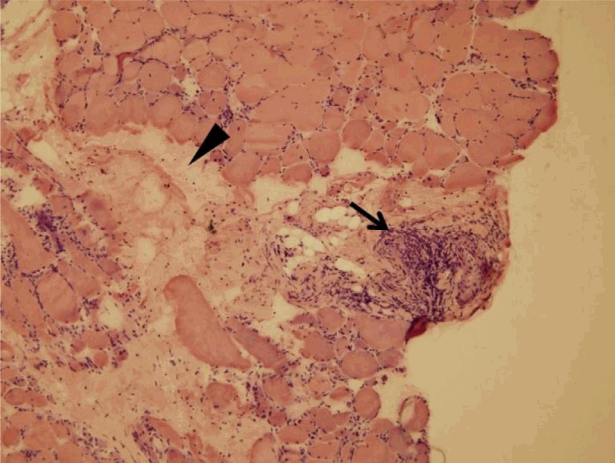
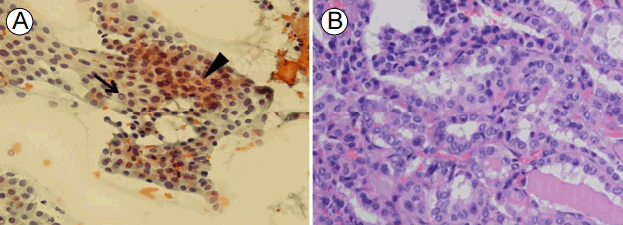
Laboratory studies revealed white blood cell count of 4,420/mm3, hemoglobin level of 11.4 g/dL, and platelet count of 312 ├Ś 103/mm3. Alanine aminotransferase level was 50 IU/L (reference: 6-40), aspartate aminotransferase level 216 IU/L (reference: 10-40), alkaline phosphatase level 73 IU/L (reference: 35-104), lactate dehydrogenase level 1,032 IU/L (reference: 135-214), total bilirubin level 0.25 mg/dL (reference: 0.1-1.2), gamma-glutamyltranspeptidase level 13 IU/L (reference: 11-73), blood urea nitrogen level 10.6 mg/dL (reference: 6-26), creatinine level 0.55 mg/dL (reference: 0.4-1.2), creatine kinase (CK) level 4,206.2 U/L (reference: 5-217), myoglobin level 444.9 ng/mL (reference: 11.1-57.5), and C-reactive protein level 0.35 mg/dL (reference: 0-0.5). Urinalysis yielded normal findings. Anti-nuclear antibody test was positive at > 1:1,280 with speckled pattern. Anti-RNP antibody level was 200.0 U/mL (reference: < 25). Anti-SS-A/Ro antibody level was 200 U/mL (reference: < 15) and anti-SS-B/La was 200 U/mL (reference: < 15). C3 complement concentration was 16 mg/dL (reference: 90-180) and C4 was 2.6 mg/dL (reference: 10-40). Rheumatoid factor level was 17.9 IU/mL (reference: 0-14). We detected no antibodies against cyclic citrullinated peptide, double-stranded DNA, centromeres, Scl-70, or Jo-1. Thyroid hormone tests revealed subclinical hypothyroidism: T3, 57.2 ng/dL (reference: 79.8-200); free T4, 1.48 ng/dL (reference: 0.75-2.00); and thyroid-stimulating hormone, 9.93 ┬ĄIU/mL (reference: 0.3-5.0). Anti-thyroid peroxidase antibody level was normal at 10.64 U/mL (reference: 0-60). Serum CK-MB level was highly elevated at 102.08 ┬Ąg/mL (reference: 0.5-5.0) and troponin I level was 0.30 ng/mL (reference: 0-0.05). T-wave inversion was evident on electrocardiogram. However, coronary angiography was normal. Transthoracic echocardiography revealed pulmonary artery pressure of 38 mm Hg, indicative of moderate, resting pulmonary arterial hypertension. Chest radiography and non-contrast-enhanced chest computed tomography revealed ground glass opacity and consolidation in the inferior regions of both the lower and left upper lobes, suggestive of nonspecific interstitial pneumonia (Fig. 2). Electromyographic data from the upper and lower limbs were compatible with the presence of active myopathy. Histological examination of biopsy specimens from the right tibialis anterior muscle revealed moderate myopathy with a few probable perivascular lymphocytic aggregations, exhibiting indistinct perifascicular atrophic features and frequent fiber degeneration and regeneration, suggestive of inflammatory myopathy (Fig. 3). We therefore confirmed a diagnosis of MCTD by using the Alarcon-Segovia criteria [5] (anti-RNP antibody positivity, synovitis, skin sclerosis, RP, and myositis). In addition, thyroid ultrasonography was performed to evaluate subclinical hypothyroidism. Two thyroid nodules of low echogenicity were found. The nodule in the right lobe was 3.6 ├Ś 2.2 mm and was calcified; the nodule in the left lobe was 6.7 ├Ś 3.4 mm. An ultrasonography-guided needle aspiration biopsy of the right nodule revealed nodular hyperplasia, and biopsy of the left nodule revealed PTC (Fig. 4A). In addition, in neck computed tomography, hypodense thyroid nodules were observed in both lobes and a 0.2-cm-diameter lymph node enlargement (left level IV) was apparent. There was no history of irradiation of the head or neck and no personal or family history of thyroid disease or cancer.
Treatment was commenced with intravenous puls methylprednisolone (500 mg daily for 3 days) followed by prednisolone 1 mg/kg/day; azathioprine was administered to deal with active myositis. Prednisolone was tapered to 5 mg/day over 6 months. Of period at the time, the CK level had declined to 492 U/L and the muscle strength had improved significantly without evidence of flare or any other symptoms of MCTD. Subsequently, the patient underwent total thyroidectomy followed by two courses of radioiodine therapy. Post-surgery pathological examination revealed papillary microcarcinomas in both lobes (Fig. 4B), and left paratracheal lymph node metastasis. No extrathyroidal extension nor vascular invasion was apparent.
DISCUSSION
We herein report a case of concurrent MCTD and PTC in a patient with underlying PBC. No significant epidemiological association between MCTD and malignancy has yet been established, but MCTD is known to be associated with other rheumatic diseases, such as DM, RA, and SLE. In several case reports, MCTD was associated with malignancies such as non-HodgkinŌĆÖs lymphoma and thymoma [6,7]; possible association between MCTD and the increased risk of cancer was also suggested in previous studies [8,9]. However, no relationship between MCTD and thyroid cancer, especially PTC, has yet been described, with the exception of a single case report by Thongpooswan et al. [8]. In addition, although PBC may be accompanied by various CTDs including SS, SSc, and SLE, an association with MCTD has been rarely reported [4]. To the best of our knowledge, this is the first case of co-existing PTC, MCTD, and PBC.
MCTD is a generalized connective tissue disorder characterized by the presence of high-titer anti-U1 RNP antibodies in combination with clinical features typical of SSc, SLE, RA, PM, and DM [1]. In our patient, polyarthritis, RP, ILD, PAH, and inflammatory myositis histologically similar to idiopathic inflammatory myopathy, were all observed at the time of diagnosis of PTC; all are common clinical features of MCTD. Among the autoimmune rheumatic diseases, DM exhibits the strongest link to malignancy, and DM can be considered to be a paraneoplastic syndrome. However, no association between MCTD and cancer (including PTC) has been established. Therefore, it remains unclear in our case whether MCTD was a paraneoplastic component of the PTC, or whether the PTC was an incidental finding. Further work is needed to explore the epidemiologic and pathogenetic association between MCTD and malignancy.
PBC is an autoimmune disease of unknown etiology, characterized by slow, progressive destruction of the small intrahepatic bile ducts. Although the liver is the primarily affected tissue, PBC may involve multiple systems and often overlaps with other autoimmune diseases. Previous studies found that more than one-third of patients with PBC had CTDs [3,4]. Especially, SS, affecting principally salivary and lacrimal glands, was reported to commonly accompany PBC [4]. Immune-mediated destruction of epithelial tissue was considered to be a pathological feature common to both PBC and SS. Two reviews found that MCTD co-existing with PBC was extremely rare [3,4], although PBC and MCTD share certain clinical features like RP, ILD, and PAH. The overall low prevalence of MCTD may explain the lack of reports on overlap between MCTD and PBC. However, the clinical and immunologic relationships between MCTD and PBC must be elucidated in future research.
PTC is the most common form of thyroid cancer, the incidence of which has increased worldwide over the last few decades. Risk factors for PTC include genetic susceptibilities, exposure to radiation or environmental pollutants, obesity, and autoimmune thyroiditis. In particular, autoimmune thyroiditis may increase thyroid cancer risk not only by increasing thyroid stimulating hormone levels, but also by stimulating autoimmune processes per se, mediated by proinflammatory cytokines and oxidative stress. Our patient lacked family history of thyroid cancer, had not been occupationally exposed to pollutants, did not have autoimmune thyroiditis, and was not obese. Given the link between chronic inflammation and malignancy, the underlying PBC may have influenced PTC development in our case. However, a recent meta-analysis found that PBC did not increase cancer risk, with the exception of hepatocellular carcinoma [10]. Thus, we found no obvious risk factor for PTC in our patient. However, based on this experience, we suggest that PTC may develop even in subjects lacking risk factors if MCTD and/or PBC are/is present.
In conclusion, this is the first report of co-existing MCTD and PTC in a patient with PBC. This highlights a possible association between MCTD and thyroid cancer, and suggests that MCTD is associated with PBC as other autoimmune diseases are. Clinicians need to monitor MCTD patients carefully for cancer. Further studies are needed to identify pathogenetic features common to MCTD and PBC.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print






