문맥 종양 혈전을 동반한 간세포암에서 방사선 치료와 면역관문억제제 병합 요법의 효과
Efficacy of Radiotherapy in Combination with Immune Checkpoint Blockade for Hepatocellular Carcinoma and Portal Vein Tumor Thrombosis
Article information
Abstract
목적
간세포암과 문맥 종양 혈전은 불량한 예후와 더불어 아테졸리주맙과 베바시주맙 병용 요법에 대한 낮은 반응률과 관련이 있다. 본 연구에서는 아테졸리주맙과 베바시주맙 병용 요법 치료를 받는 간세포암 환자에서 방사선 치료의 안전성과 유효성을 평가하고자 하였다.
방법
이 후향적 코호트 연구는 한국의 단일 3차 의료기관에서 간세포암과 문맥 종양 혈전을 진단받은 환자를 대상으로 하였다. 대상자는 Child-Pugh A 등 급의 간기능을 보유하고 ECOG 수행능력 점수가 1 이하이며 수술적 절제나 국소 치료의 적응증이 없는 경우였다. 무진행 생존 및 전체 생존의 예측 인자를 확인하기 위하여 Cox 회귀분석을 시행하였다.
결과
총 85명의 환자가 포함되었으며 이 중 55명은 아테졸리주맙과 베바시주맙 병용 요법 단독, 30명은 아테졸리주맙과 베바시주맙 병용 요법과 추가 방사선 치료를 받았다. 두 군 간 질병 조절률(25% vs. 30%, p= 0.65), 무진행 생존(중앙값, 143일 vs. 289일; p= 0.17), 전체 생존(중앙값, 293일 vs. 416일; p= 0.16)에는 유의한 차이가 없었다. 그러나 주간문맥 침범 환자를 제외한 하위군 분석에서 병합 치료는 무진행 생존(aHR, 0.29; p= 0.002)과 전체 생존(aHR, 0.33; p= 0.01)을 유의하게 향상시켰다. 또한 방사선 치료 시행 후 임상적 혹은 간기능 관련 혈액 검사 악화는 관찰되지 않았다.
결론
간기능이 보존되고 주간문맥 침범이 없는 환자에서 아테졸리주맙과 베바시주맙 병용 요법과 병행한 문맥 종양 혈전 대상 방사선 치료는 잠재적으로 유용할 수 있다.
Trans Abstract
Background/Aims
Hepatocellular carcinoma (HCC) with portal vein tumor thrombosis (PVTT) is associated with poor prognosis and low response rates to standard atezolizumab plus bevacizumab therapy (atezo/bev). This study aimed to evaluate the safety and efficacy of radiation therapy (RT) in patients with HCC and PVTT receiving atezo/bev treatment.
Methods
This retrospective cohort study included patients with HCC and PVTT treated at a tertiary referral center in Korea. Eligible patients had Child-Pugh class A liver function and an Eastern Cooperative Oncology Group performance status of ≤ 1, and were not candidates for surgical resection or loco-regional therapies. Cox regression analysis was used to identify predictors of progression-free survival (PFS) and overall survival (OS).
Results
A total of 85 patients were included, 55 of whom received atezo/bev alone and 30 received atezo/bev combined with RT. There was no significant difference in disease control rate between the two groups (25% vs. 30%, p = 0.65), nor in PFS or OS (median PFS, 143 vs. 289 days, p = 0.17; median OS, 293 vs. 416 days, p = 0.16). However, subgroup analysis, excluding patients with main portal trunk invasion, showed that the addition of RT significantly improved both PFS (adjusted hazard ratio [aHR], 0.29; p = 0.002) and OS (aHR, 0.33; p = 0.01). No clinical or laboratory deteriorations were observed after RT.
Conclusions
These findings suggest that the addition of RT to PVTT may be beneficial in patients with preserved liver function without main portal vein involvement.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third leading cause of cancer-related deaths, with an estimated 906,000 new cases and 830,000 deaths worldwide in 2020 [1]. Portal vein tumor thrombosis (PVTT) is a frequent complication of HCC that is associated with aggressive tumor biology and poor prognosis, with a median survival of only a few months [2-4]. PVTT is classified as an advanced-stage (Barcelona Clinic Liver Cancer [BCLC] stage C) and is typically not amenable to locoregional treatment. Systemic therapy is the first line of treatment for patients with PVTT [5,6]. Multikinase inhibitors, such as sorafenib and lenvatinib, are the mainstay systemic agents that provide modest survival benefits for patients with BCLC stage C disease. However, pooled analyses of randomized sorafenib trials have shown that major vascular invasion is associated with poor survival in all patient subgroups [7]. Although preliminary studies have suggested that lenvatinib may yield better outcomes than sorafenib in patients with PVTT [8], a randomized phase III trial in patients with HCC found no significant difference in overall survival between the two drugs in the presence of PVTT [9].
The phase III IMbrave150 trial demonstrated that the combination of atezolizumab, an immune checkpoint inhibitor (ICI) targeting programmed death-ligand 1, and bevacizumab, a monoclonal antibody targeting vascular endothelial growth factor, significantly improved survival outcomes in patients with advanced HCC compared to sorafenib [10]. Therefore, atezolizumab plus bevacizumab (atezo/bev) has been established as the first-line treatment for advanced HCC [5,6]. Updated data from the IMbrave150 trial showed that the superior efficacy of atezo/bev over sorafenib was maintained, regardless of the presence of PVTT [11], suggesting that patients with advanced HCC and PVTT can also benefit from atezo/bev therapy. Nevertheless, response rates remain low, with only about one-quarter of patients with PVTT achieving an objective response to atezo/bev [11].
Since HCC is a radiosensitive malignancy; therefore, external beam radiotherapy (EBRT) has been clinically employed for the local control of PVTT [12]. Advances in imaging and radiation delivery have made radiation therapy (RT) increasingly feasible for patients with PVTT who often have limited hepatic reserve [13]. A recent meta-analysis reported an objective response rate (ORR) of 50.4% for conventional EBRT, either alone or in combination with other treatment modalities [14]. Current guidelines from the American Society for Radiation Oncology conditionally recommend EBRT as a treatment option for HCC with macrovascular invasion, either as a monotherapy or in sequence with systemic or catheter-based therapies [15].
The combination of radiation and ICIs has been actively investigated for several solid tumors [16]. In HCC, phase I/II trials combining ICIs with EBRT have demonstrated promising survival outcomes and acceptable safety profiles, including those in patients undergoing PVTT [17-20]. Recent small studies using combined ICIs and intensity-modulated radiotherapy (IMRT) for PVTT reported variable response rates (ORR, 49-77%) [21-23]. However, it remains unclear whether the addition of EBRT enhances the efficacy of atezo/bev therapy in patients with HCC and PVTT. Therefore, the aim of this study was to evaluate the efficacy and safety of EBRT in HCC patients with PVTT receiving atezo/bev treatment.
MATERIALS AND METHODS
Study populations
This single-center retrospective cohort study included all consecutive HCC patients with PVTT aged 18 years or older who received atezo/bev as first-line systemic therapy at our institution between May 2022 and December 2023. Eligible patients had stage III or higher disease, Child-Pugh class A liver function, and an Eastern Cooperative Oncology Group performance status (ECOG PS) of ≤ 1. Patients were excluded if they were candidates for surgical resection or locoregional therapy. HCC was diagnosed based on histopathology or diagnostic criteria outlined in the American Association for the Study of Liver Diseases guidelines [6]. PVTT was defined as an intraluminal filling defect in the portal venous system identified on contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI). PVTT was classified according to the Japanese Vp classification system [24]: Vp0, no PVTT. Vp1, PVTT distal to, but not involving, the second-order portal vein branches. Vp2, PVTT invading the second-order branches. Vp3, PVTT present in the first-order branches. Vp4, PVTT extending into the main portal trunk and/or contralateral portal vein branches.
Treatments
Atezolizumab (1,200 mg) and bevacizumab (15 mg/kg) were administered intravenously every 3 weeks until disease progression or the development of unacceptable toxicity. The decision to administer EBRT in combination with atezo/bev was made after a multidisciplinary review by hepatologists, medical oncologists, and radiation oncologists. The type of treatment (IMRT or stereotactic body radiotherapy [SBRT]), dosing, and fractionation schedule were determined at the discretion of the radiation oncologist based on the patient’s clinical status, tumor volume, and proximity to critical organs at risk (Supplementary Table 1). CT simulation with contrast enhancement was performed with the patient in the supine position using immobilization devices and motion management techniques. Contouring and treatment plans were reviewed and approved during the daily chart rounds. The patients were evaluated weekly throughout the course of irradiation. Treatment planning was performed using Eclipse software (Varian, Palo Alto, CA, USA), and radiation was delivered using TrueBeam® and VitalBeam® systems (Varian).
Assessment of outcomes
Dynamic contrast-enhanced CT or MRI was used to assess treatment efficacy after every two cycles of atezo/bev. Tumor response was evaluated using the modified Response Evaluation Criteria in Solid Tumors (mRECIST) [25]. For PVTT, responses were classified according to previously reported criteria [26]: partial response (PR) was defined as downstaging in the PVTT classification, progressive disease (PD) was defined as upstaging in the PVTT classification, and stable disease (SD) was defined as no change in the PVTT classification (i.e., neither PR nor PD). The overall response was defined as follows: PD, if there was any progression of the primary liver tumor, PVTT, or extrahepatic lesions. PR, if any of these components showed a partial response without evidence of progression elsewhere, and SD in all other cases. Progression-free survival (PFS) was calculated from the date of atezo/bev initiation to the date of disease progression as defined above. Overall survival (OS) was calculated from the date of atezo/bev treatment initiation to the date of death. The ORR was defined as the percentage of patients achieving a confirmed complete or PR. The disease control rate (DCR) was defined as the proportion of patients who achieved either an objective response or SD.
Data collection
Data were collected from the clinical data warehouse (CDW) and electronic medical records (EMR). The CDW contains comprehensive data on each patient encounter including diagnostic codes (ICD-10), radiological reports, laboratory results, and pharmacy information. EMRs were used to extract demographic information (age, sex), alcohol use history, ECOG PS, confirmation of diagnoses (including pre-treatment tumor burden, liver cirrhosis status, and baseline Charlson comorbidity index), as well as to monitor clinical progression and verify outcome data.
Vital status, including the date and cause of death, was obtained from the EMR records and Statistics Korea via the Korean Statistical Information Service database.
Statistical analysis
Statistical analyses were performed using the STATA software (version SE/14.0; StataCorp LLC, College Station, TX, USA). Group comparisons were conducted using Student’s t-test or the Mann-Whitney-Wilcoxon test for continuous variables and Pearson’s chi-squared test for categorical variables.
Univariate and multivariate Cox proportional hazards models were used to identify independent prognostic factors for survival, and the results are reported as hazard ratios (HRs). Statistical significance was set at p< 0.05. Kaplan-Meier survival curves were generated for PFS and OS, with HRs and log-rank p-values calculated and displayed. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (No. B-2404-892-103) and was conducted in accordance with the principles of the Declaration of Helsinki. The requirement for written informed consent was waived because the data contains anonymized information that complies with the guidelines of the Personal Data Protection Act.
RESULTS
Patient characteristics
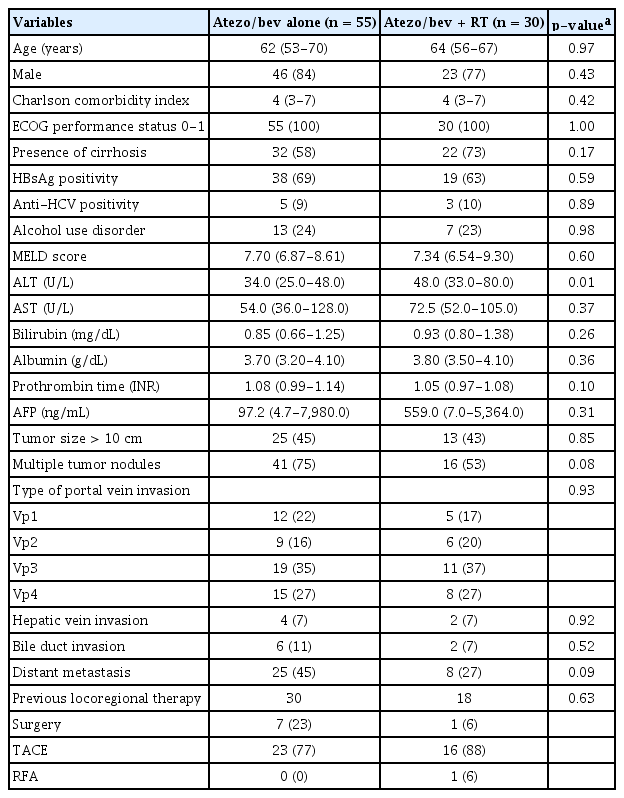
Among 202 patients with HCC received atezo/bev as initial systemic therapy, 102 (51%) had PVTT identified on baseline CT or MRI prior to treatment initiation. After excluding 17 patients who were lost to follow-up without imaging assessment, 85 patients were included in the final analysis and were defined as the overall cohort (Fig. 1). Of these, 55 received atezo/bev alone and 30 received atezo/bev combined with RT.
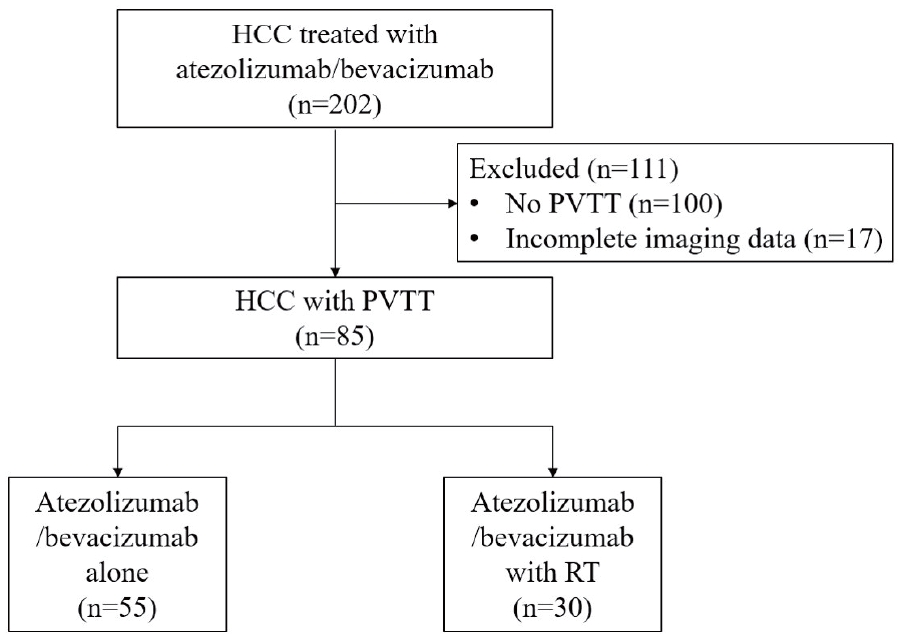
Flow diagram of patients included in the analysis. HCC, hepatocellular carcinoma; PVTT, portal vein tumor thrombosis; RT, radiation therapy.
The baseline characteristics of the study population are summarized in Table 1. The cohort had a mean age of 62 years (SD, 10 years), and 69 patients (81%) were male. The clinical and laboratory parameters were comparable between the two groups, except for serum alanine aminotransferase (ALT) levels, which were significantly higher in the atezo/bev plus RT group than in the atezo/Bev group (p= 0.01).
The distribution of portal vein invasion according to the Japanese Vp classification did not differ significantly between the two groups (p= 0.93). Vp3 was the most common type of PVTT in both groups, observed in 35% and 37% of the patients, respectively. A schematic representation of the PVTT classification is shown in Supplementary Fig. 1.
Treatment efficacy
The DCR, assessed using the mRECIST criteria, was 27% in the overall cohort and did not differ significantly between the groups: 25% (14 of 55 patients) in the atezo/bev group vs. 30% (9 of 30 patients) in the atezo/bev + RT group (p= 0.65).
The median PFS for the entire cohort was 183 days (interquartile range [IQR], 63-367). Patients in the atezo/bev group had a median PFS of 143 days (IQR, 60-309), whereas those in the atezo/bev plus RT group had a median PFS of 289 days (IQR, 133-506) (log-rank, p= 0.17) (Fig. 2).
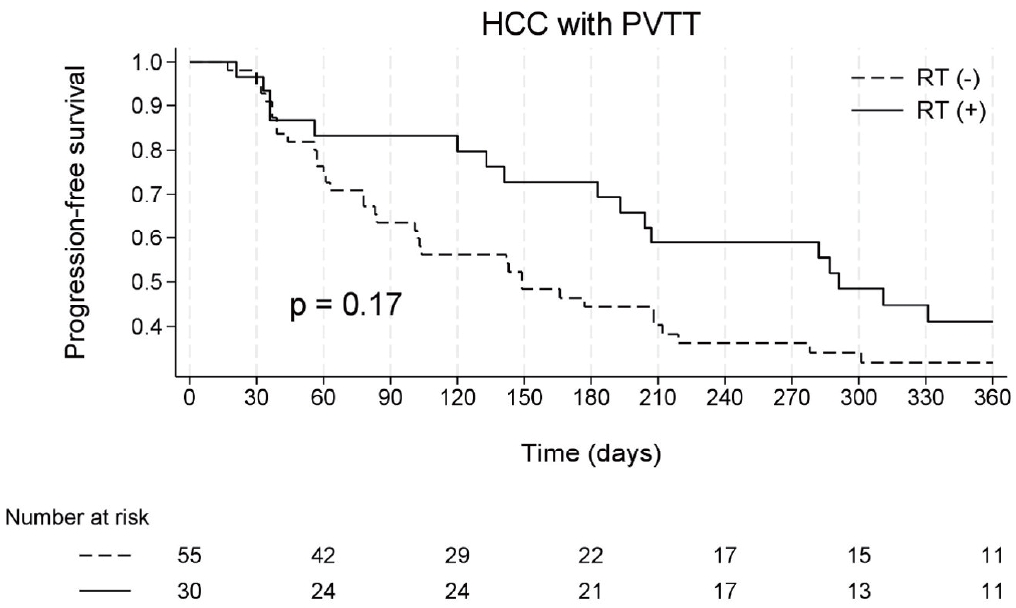
Kaplan-Meier estimates of progression-free survival, defined as time from atezolizumab plus bevacizumab initiation to disease progression. HCC, hepatocellular carcinoma; PVTT, portal vein tumor thrombosis; RT, radiation therapy.
Univariate analysis identified the female sex, elevated serum aspartate transaminase (AST) and bilirubin levels, and hepatic vein invasion as significant predictors of disease progression (Supplementary Table 2). However, in the multivariate analysis, none of the evaluated covariates, including the addition of RT, remained statistically significant (HR = 0.69, p= 0.17 in univariate analysis; adjusted HR [aHR] = 0.63, p= 0.10 in multivariate analysis).
All the deaths recorded during the study period were attributable to liver-related causes. The median OS, defined as the time from the initiation of atezo/bev treatment to death, was 333 (IQR, 148-502) for the entire cohort. Median OS was 293 (IQR, 129-434) in the atezo/bev group and 416 (IQR, 193- 595) in the atezo/bev + RT group (log-rank test, p= 0.16) (Fig. 3).
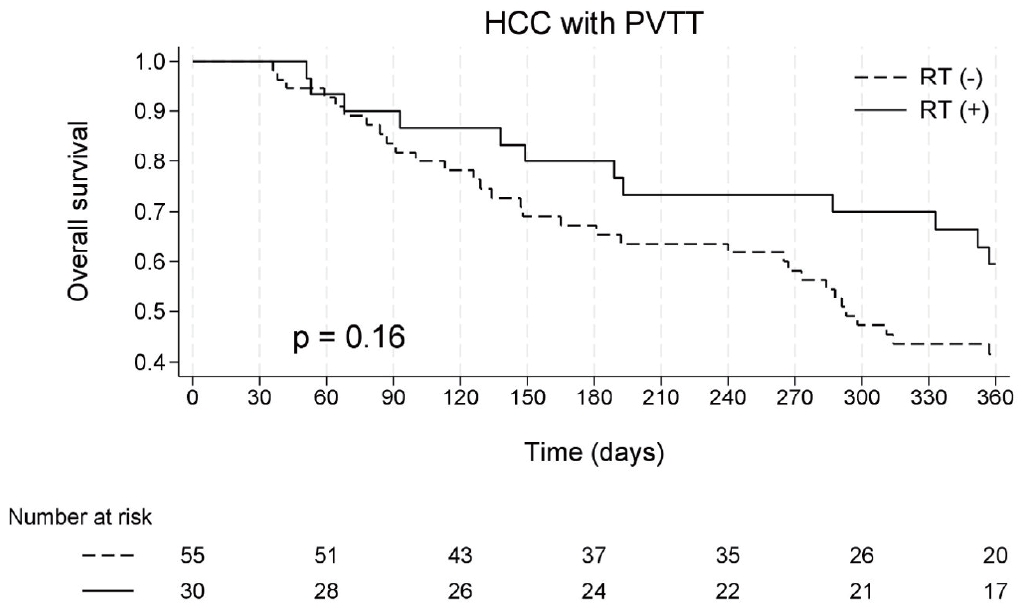
Kaplan-Meier estimates of overall survival, defined as time from atezolizumab plus bevacizumab initiation to death. HCC, hepatocellular carcinoma; PVTT, portal vein tumor thrombosis; RT, radiation therapy.
In the univariate analysis, the predictors of mortality included elevated bilirubin and AST levels, and lower serum albumin levels. However, in multivariate analysis, the addition of RT did not significantly affect the risk of death (aHR = 0.71, p= 0.25) (Supplementary Table 3).
Subgroup analyses in patients without involvement of the main portal trunk (Vp1-3)
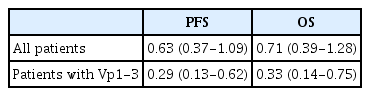
We conducted subgroup analyses of PFS, OS, and DCR in 62 patients without Vp4 involvement, because Vp4 invasion is associated with extremely poor prognosis and rapid disease progression. Among these patients, the overall DCR was 31% (19 of 62): 30% (12 of 40) in the atezo/bev-only group and 32% (7 of 22) in the group that received systemic therapy plus RT. Multivariate Cox regression analysis revealed that the addition of RT was significantly associated with an improved PFS (aHR = 0.29, p= 0.002) and OS (aHR = 0.33, p= 0.01) (Table 2; Supplementary Tables 4, 5; Supplementary Figs. 2, 3).
Safety of radiation for PVTT
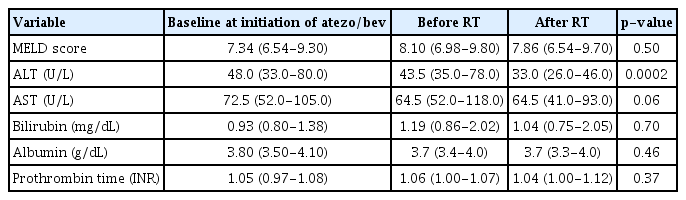
No major signs, symptoms, or deterioration in the ECOG PS were observed, which could be attributed to RT during or after the treatment period. Follow-up laboratory assessments demonstrated preserved liver function, including stable model for end-stage liver disease scores, and a significant reduction in serum ALT levels (43.5 vs. 33.0, p= 0.0002) after RT (Table 3).
DISCUSSION
HCC with PVTT remains a major clinical challenge because of its potential for microscopic dissemination. Even limited portal vein involvement can preclude curative-intent treatments, whereas invasion of the main trunk or first-order branches is associated with poor prognosis and rapid disease progression [27]. Although ICIs have demonstrated improved treatment outcomes compared with tyrosine kinase inhibitors (TKIs), the overall response rates remain suboptimal [7,9]. Given the limitations of the current systemic therapies for PVTT, combining locoregional and systemic treatments may yield synergistic effects by addressing both rapid local progression and multifocal tumor growth. However, conventional EBRT, which is constrained by the risk of radiation-induced liver injury, has shown response rates of less than 30% [28]. In contrast, SBRT and IMRT have demonstrated improved tumor control and favorable toxicity profiles [15]. Therefore, these modalities may serve as effective adjuncts to systemic therapy for PVTT.
Historically, RT has been explored as a strategy to enhance the limited efficacy of standard TKI-based treatments for PVTT [29,30]. These observed synergistic anticancer effects may be partially explained by the ability of sorafenib to modulate the radiosensitivity of HCC cells [31,32]. Moreover, ionizing radiation can stimulate both local and systemic immune-mediated antitumor responses, suggesting that EBRT may potentiate the effects of ICIs [16,17,33]. Recent case series and single-arm studies have reported promising outcomes of combined radiation and immunotherapy in patients with PVTT [18,22,34,35]. Despite the increasing role of EBRT as a primary radiation modality, there is a lack of retrospective or prospective studies specifically evaluating the combination of EBRT with atezo/bev, the current first-line treatment for unresectable HCC.
This single-center retrospective study evaluated the effect of EBRT in patients receiving atezo/bev therapy and found that the addition of radiotherapy was not associated with a significant survival benefit across all types of PVTT. However, when patients with Vp4 invasion were excluded, the combination of EBRT with systemic therapy was associated with significantly improved PFS and OS compared to atezo/bev alone.
Previous studies have shown that both the presence and extent of PVTT significantly influence patient outcomes. Among the PVTT classifications, Vp4, which is defined as a tumor thrombus extending into the main portal vein trunk, is associated with the poorest prognosis. This subtype corresponds to the lowest median OS of approximately 4.3 months among patients with HCC and PVTT, underscoring the aggressive nature of the disease at this stage [2,36]. Although limited data exist on the effect of combining RT with systemic therapy in Vp4 cases, the aggressive behavior and rapid progression associated with Vp4 PVTT may limit the efficacy of such combination treatments relative to those in patients with less extensive involvement. The poor prognosis of Vp4 patients may have masked any potential benefits of RT in the overall cohort, highlighting the need for future studies specifically designed for this subgroup. Although this study could not elucidate the mechanistic reasons why additional RT failed to improve clinical outcomes in cases of main trunk invasion, our findings support the selection of appropriate candidates for adjunctive radiotherapy during atezo/bev treatment.
Several mechanisms of synergy between RT and ICIs have been proposed for the treatment of locally advanced solid tumors [33]. First, RT induces immunogenic cell death, leading to the release of tumor-associated antigens that enhance immune recognition and stimulate a robust immune response. Radiation can also upregulate major histocompatibility complex molecules in tumor cells, thereby improving antigen presentation to T cells and promoting stronger T cell activation. Additionally, RT contributes to the proinflammatory tumor microenvironment by releasing cytokines and chemokines that attract immune cells to the tumor site. These mechanisms may also contribute to the abscopal effect, a phenomenon in which localized radiation induces systemic immune responses that reduce the size of distant untreated tumors. Finally, RT may mitigate the immunosuppressive tumor microenvironment, thereby enhancing the therapeutic effects of ICIs. Whether the benefits of additional RT observed in our study reflect these immunogenic mechanisms remains to be elucidated in future research.
Radiation-induced liver injury is a major concern in the use of RT for liver tumors because it can be dose limiting and, in severe cases, can lead to liver failure or death. In the present study, we observed no significant deterioration in hepatic function during EBRT. However, as all patients had well-compensated liver function at baseline (Child-Pugh class A), it remains uncertain whether similar safety and efficacy would apply to patients with decompensated liver disease. Our findings suggest that RT can be safely administered to patients with preserved liver function, particularly when the PVTT is confined to the second-order or more distal portal vein branches.
This study has several limitations. First, owing to its single-center retrospective design and limited sample size, we were unable to establish a causal relationship between combination therapy and observed survival outcomes. This study was also subject to potential selection bias and unmeasured confounding factors. The administration and dosing of RT were determined at the discretion of individual physicians based on varying clinical conditions without a standardized treatment protocol. This variability may have further contributed to the selection bias. For example, the lower baseline ALT levels in the atezo/bev only group suggest better preservation of liver function, indicating a potential physiological advantage at baseline and a potential bias in the selection of patients for RT. Nevertheless, the observation that the atezo/bev group, despite having higher baseline ALT levels, demonstrated superior outcomes supports the potential added therapeutic benefit of RT, which may outweigh the baseline disadvantages.
Second, although the two treatment groups were generally well balanced in terms of baseline characteristics, the small sample size may have limited the statistical power to detect significant differences in PFS or OS. Based on previously reported survival data from larger studies [10,37], detecting a HR of 0.67 for OS, would require approximately 280 patients to achieve 80% power at a 5% significance level. The small sample size in our study increased the likelihood of a type II error, potentially masking the true treatment effect.
Third, while the positive outcomes observed in patients with Vp1-3 PVTT were encouraging, these findings were derived from a subgroup analysis that excluded patients with Vp4 involvement. Therefore, these results should be interpreted with caution when considering the efficacy of combination therapy in a broader population of patients with PVTT. Additionally, the relatively short follow-up duration limited our analysis to 1-year survival outcomes. This abbreviated observation period may have prevented the detection of long-term changes in local tumor characteristics post-RT, hindering a more comprehensive understanding of the mechanistic effects of RT.
Finally, the study population was heterogeneous with respect to prior locoregional therapy. Although the presence or absence of such treatments was not a significant predictor of survival in the multivariate analysis, we were unable to determine whether treatment-naïve patients with PVTT responded differently than those who experienced progression after prior locoregional interventions.
In conclusion, this study found that the addition of RT did not significantly improve the tumor response or survival in an overall cohort of HCC patients with PVTT receiving atezo/bev systemic therapy. However, subgroup analysis revealed improved PFS and OS in patients whose PVTT was limited to Vp1-3. These findings support the hypothesis that, in the absence of main portal vein invasion, the addition of RT may offer clinical benefits when combined with atezo/bev therapy in this patient population. Further prospective studies with larger cohorts and longer follow-up periods are required to validate these results and address the limitations discussed above.
Supplementary Materials
Cox regression analysis of risk factors for HCC progression in the overall patients (Vp1-4) (n = 85)
Cox regression analysis predicting death in the overall patients (Vp1-4) (n = 85)
Cox regression analysis of risk factors for HCC progression in patients with Vp1-3 (n = 62)
Alluvial plot showing response patterns based on dose of radiation therapy. PVTT, portal vein tumor thrombosis; RT, radiation therapy; mRECIST, modified Response Evaluation Criteria in Solid Tumors; PD, progressive disease; SD, stable disease.
Notes
CONFLICTS OF INTEREST
No potential conflicts of interest relevant to this article were reported.
FUNDING
This study was not supported by any sponsor or funder.
AUTHOR CONTRIBUTIONS
All authors contributed substantially and in accordance with the guidelines of the International Committee of Medical Journal Editors. Study concept and design: YL, EJ, GC, SJ, JWK, MK, JHK, CS, and JWK. Acquisition, analysis, or interpretation of data: YL, EJ, GC, SJ, JWK, MK, JHK, CS, and JWK. Drafting the work or revising: YL, EJ, GC, SJ, JWK, MK, JHK, CS, and JWK. Final approval of the manuscript: YL, EJ, GC, SJ, JWK, MK, JHK, CS, and JWK.
ACKNOWLEDGEMENTS
None.