Extended-Spectrum β-Lactamase 생성 장내세균속균종 균혈증 환자들의 치료에서 비카바페넴 항생제의 단일 기관 치료 결과: Piperacillin-Tazobactam을 중점으로
Non-carbapenem Drugs for Patients with Bacteremia caused by Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae: Piperacillin-Tazobactam
Article information
Abstract
목적
장내세균에서 3세대 cephalosporin 계열 항생제에 내성을 나타내는 주된 내성 기전은 extended spectrum β-lactamase (ESBL)를 생성하는 것이다. ESBL 생성 장내세균은 ESBL 생성균이라고 하더라도 piperacillin/tazobactam 감수성 균인 경우 piperacillin/tazobactam을 사용해 볼 수 있으며 후향적 연구에서 carbapenem 계열 항생제를 투여한 경우에 비해 열등하지 않은 치료 효과가 보고된 바 있다. 하지만 미국감염학회 2023년 지침에서는 ESBL 생성 그람음성균 감염에서 carbapenem 계열 항생제를 권고하고 piperacillin/tazobactam 감수성인 그람음성균이더라도 carbapenem 계열 항생제를 투여하도록 하고 있다. 하지만 carbapenem 내성 장내세균의 증가 등으로 비 carbapenem 계열의 항생제들의 사용 필요성에 대한 평가는 아직도 필요한 상황이다. 이에 제주대학교병원의 ESBL 생성 장내세균속 균혈증 환자들의 carbapenem과 비 carbapenem 계열 항생제의 치료 효과를 보고한다.
방법
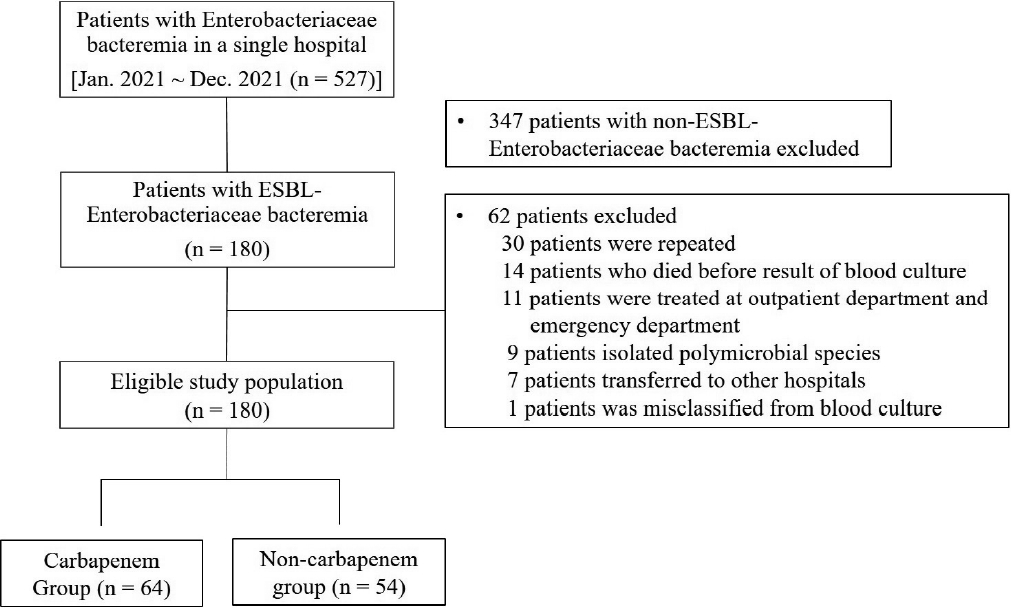
2021년 1월부터 12월까지 527명의 성인 장내세균속 균혈증 환자들 중에서 ESBL 생성 장내세균속 환자 152명을 대상으로 후향적 자료를 수집하고 3개월 이내 장내세균 감염이 있었던 환자들과 30일 후 추적 관찰을 하지 못한 환자들을 제외하고 분석하였다.
결과
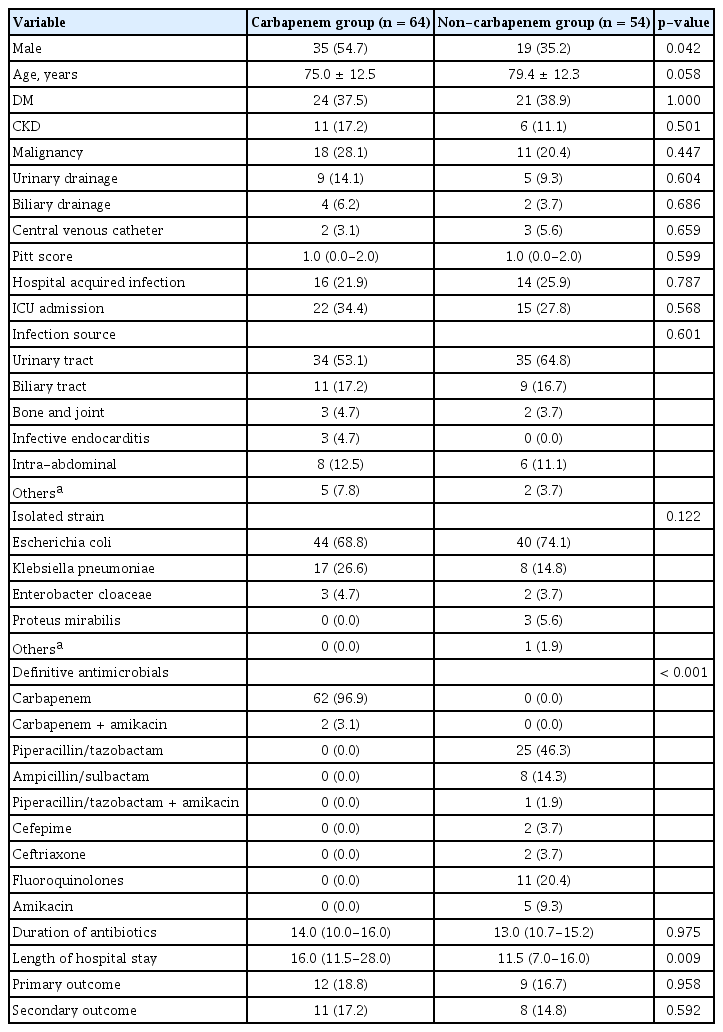
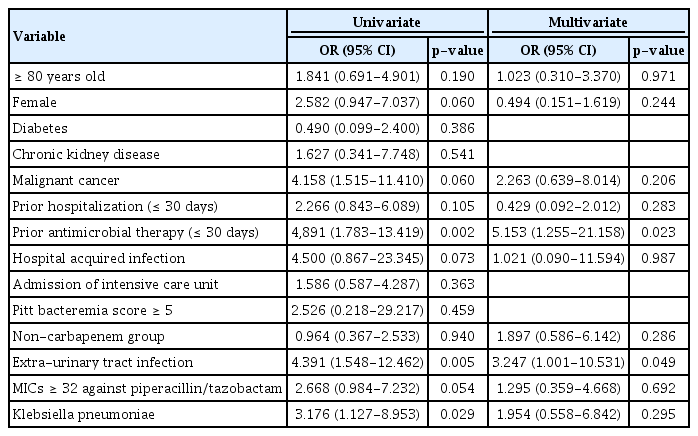
총 118명의 ESBL 생성 장내세균속 균혈증 환자들 중에서 54명의 환자가 확정적 항생제로 비 carbapenem 계열 항생제로 치료하였고 64명의 환자가 carbapenem 계열 항생제로 치료하였다. Kaplan-Meier 생존 분석 방법으로 30일 치료 실패율과 사망률을 분석한 결과 30일 치료 실패율은 비 carbapenem군이 16.7%, carbapenem군이 18.8%로 양 군 간의 유의한 차이는 없었다(p= 0.65). 30일 사망률은 비 carbapenem 계열군이 14.8%, carbapenem 계열군이 17.2%로 양 군 간의 유의한 차이는 없었다(p= 0.63). 전체 환자들 중에서 치료 실패한 환자들을 다변량 로지스틱 회귀 분석한 결과에서 요로 감염 외 감염과 이전 30일 이내 항생제 사용력이 있는 경우 치료 실패의 위험인자로 확인되었다.
결론
ESBL 생성 장내세균속 균혈증 환자들은 국내에서 확정적 항생제로 비 carbapenem 계열의 항생제를 사용해 볼 수 있으나 중증 환자들을 포함한 다기관, 전향적 후속 연구들이 필요하다.
Trans Abstract
Background/Aims
Carbapenems are recommended for treating bacteremia caused by extended-spectrum β-lactamase (ESBL) producing Enterobacteriaceae (ESBL-E). However, this has resulted in a significant rise in the utilization of carbapenems in cases of ESBL-E infection. We evaluated the clinical outcomes of patients with ESBL-E bacteremia treated with non-carbapenem antimicrobials.
Methods
We conducted a retrospective case-control study of a cohort of patients with documented ESBL-E bacteremia from January 2021 to December 2021. The patients were divided into two groups according to whether they received non-carbapenem or carbapenem therapy. The rates of treatment failure, 30-day mortality and microbiologic failure, and the durations of hospitalization and of antimicrobial therapy were compared between the two groups. Antimicrobial susceptibility testing and phenotypic identification of ESBL-E were performed using the Vitek 2 system.
Results
Of 118 patients with ESBL-E bacteremia, 54 received non-carbapenem drugs (non-carbapenem group [NCG]) and 64 received carbapenems (carbapenem group [CG]). Treatment failure at 30 days occurred in 16.7% of the patients in the NCG and in 18.8% in the CG (p = 0.65). The 30-day mortality rate was 14.8% in the NCG and 17.2% in the CG (p = 0.63). Extra-urinary tract infection and prior antimicrobial therapy within 30 days were risk factors for treatment failure in patients with ESBL-E bacteremia. The clinical outcomes did not differ significantly between the two groups, challenging the prevailing preference for carbapenems in the treatment of ESBL-E bacteremia.
Conclusions
Non-carbapenem antimicrobials such as piperacillin/tazobactam are recommended for patients with mild ESBL-E bacteremia in South Korea.
INTRODUCTION
Extended-spectrum β-lactamases (ESBL), first described in 1983, are inhibited by clavulanic acid, sulbactam, and tazobactam. In 1991, cefotaxime-hydrolyzing β-lactamase-Munich (CTX-M-1) ESBLs were identified [1]. The increasing prevalence of antimicrobial resistance, particularly ESBL-producing Enterobacteriaceae (ESBL-E), is a threat to health globally. The incidence of ESBL-E identified in bacterial cultures increased by 53% in the United States from 2012 to 2017 [2]. Between 2015 and 2019, the global prevalence of ESBL non-carbapenem-resistant Enterobacteriaceae (CRE) phenotypes was > 50% [3]. Since 2019, the prevalence of ESBL-E in South Korea has been ≥ 50% [4]. Because of the spread of ST131 with CTX-M-15, the community prevalence of ESBL has increased [5]. ESBL-E-induced bacteremia is associated with an increased hospitalization duration, adding to the clinical and economic burden of bacteremia. In a 2020 multicenter retrospective study of patients with ESBL or CRE Klebsiella pneumoniae and Escherichia coli (EC) bacteremia in South Korea, the 30-day mortality rate of ESBL-E bacteremia was 10.3%, with a treatment failure rate of 12.5%, and a total cost for medical expenses of up to $11,151 [6]. According to a guidance from the Infectious Diseases Society of America (IDSA), carbapenems are the preferred treatment options for pyelonephritis and complicated urinary tract infection caused by ESBL-E [7]. In vitro, piperacillin-tazobactam (PIP/TAZ) is effective in the treatment of urinary tract infections caused by ESBL-EC [8]. The rate of ESBL-EC has increased in community-acquired urinary tract infections [9], and although carbapenems are recommended for ESBL-E bacteremia, it is important to note that the rate of CRE continues to increase worldwide in the coronavirus disease 2019-endemic era [10]. Therefore, we evaluated the clinical outcomes of non-carbapenem drugs in patients with ESBL-E bacteremia.
MATERIALS AND METHODS
Study design
We conducted a retrospective case-control study involving adult patients with documented ESBL-E bacteremia using the electronic medical records of a teaching hospital. Between January 1, 2021 and December 30, 2021, 527 adults (≥ 18 years of age) with Enterobacteriaceae bacteremia were enrolled in the study. Patients were excluded if they had been infected with the same species (or ESBL-E) 3 months prior to the current positive result or if two or more species were identified in a specimen, including blood. Based on the exclusion criteria, 152 patients with ESBL-E bacteremia were included in the study (Fig. 1). This study was approved by the Institutional Review Board of Jeju National University Hospital (No. 2021-03-013-001).
The patients were divided into two groups according to whether they received non-carbapenem or carbapenem therapy. The rates of treatment failure, 30-day mortality and microbiologic failure, and the duration of hospitalization and of antimicrobial therapy were compared between the two groups. Antimicrobial susceptibility testing and phenotypic identification of ESBL-E were performed using the Vitek 2 system (BioMérieux, Marcy-l'Étoile, France). ESBL-E was defined as the presence of ESBL positivity or resistance to cefotaxime.
Definitions
An episode of bacteremia was defined as the 14-day period from the time of collection of the first blood culture positive for Enterobacteriaceae. Severity of acute illness was assessed at the time of positive blood culture using the Pitt bacteremia score (PBS) [11]. The primary outcome was isolation of the same species as the initial isolate from blood culture at 2 to 5 days after starting definitive antimicrobial drug maintenance, or relapsed fever, or deterioration of the clinical course leading to a change in antimicrobials 5 days after maintaining definitive antimicrobials. The secondary outcome was the 30-day mortality rate. Nosocomial infection was defined as infection acquired after hospitalization that manifested 48 hours after admission. Follow-up blood culture results were obtained at least 48 hours after administration of antimicrobial drugs.
Statistical analysis
Categorical variables were compared using the chi-squared test, and continuous variables using the two-sample t-test and analysis of variance. Continuous variables are presented as means ± standard deviations or medians and interquartile ranges. The primary and secondary outcomes at 30 days were estimated using Kaplan-Meier curves for time-to-event analysis, measured from the day of admission to the occurrence of the event during follow-up. Multivariate logistic regression analysis was performed on risk factors with values of p< 0.2, associated with treatment failure in univariate analyses. Hazard ratios (HRs) and 95% confidence intervals (CIs) were computed by Cox regression analysis. A value of p< 0.05 was considered indicative of statistical significance. Statistical analysis was performed using SPSS Statistics for Windows (ver. 22.0; IBM, Armonk, NY, USA) and R statistics (ver. 4.2.1; The R Foundation, Vienna, Austria).
RESULTS
Clinical characteristics of patients with ESBL-E bacteremia
A total of 118 patients with ESBL-E bacteremia were included in the study; their clinical characteristics are listed in Table 1. Among the patients, 54 (45.8%) were males, with a mean age of 71.1 ± 11.6 years. The overall 30-day inpatient mortality rate was 16.1%. Of the patients, 54 and 64 were classified into the non-carbapenem group (NCG) and carbapenem group (CG), respectively. The proportion of male patients was higher in the CG than in the NCG (54.7% vs. 35.2%, respectively; p= 0.042). The mean age in the NCG showed a trend toward being higher than in the CG (79.4 ± 12.3 vs. 75.0 ± 12.5, respectively; p= 0.058). There were no significant differences in the PBS or other baseline clinical characteristics between the two groups. Urinary tract infection was the most prevalent primary infection type in both of the groups. The most frequently isolated strain was EC (74.1% in the NCG vs. 68.8% in the CG; p= 0.122). Hospitalization duration was significantly longer in the CG than the NCG.
Primary and secondary outcomes
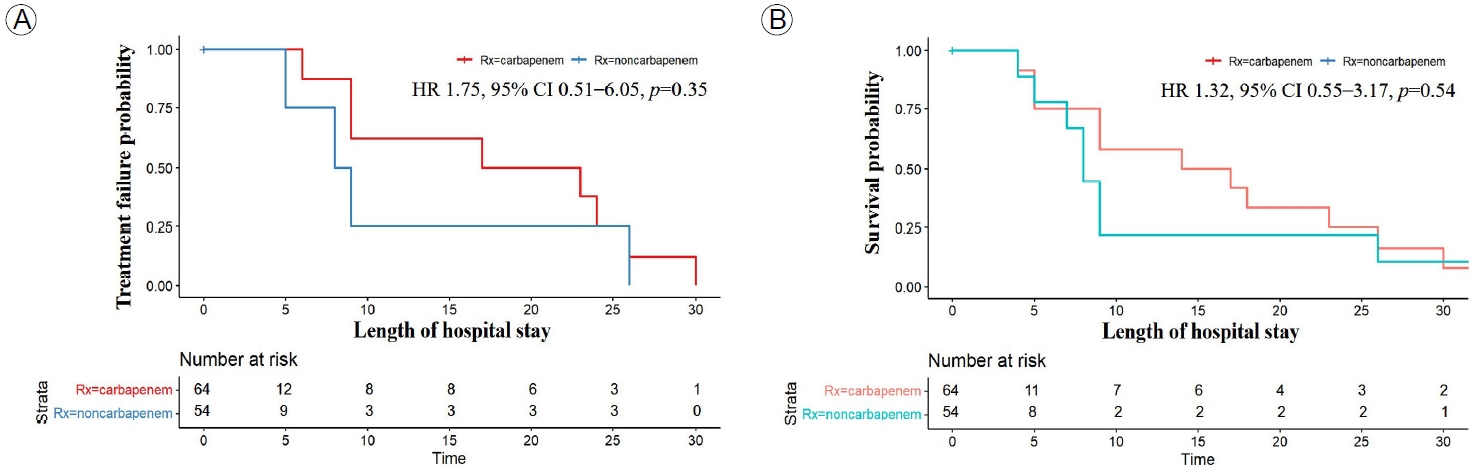
There was no significant difference in primary and secondary outcomes between the CG and NCG (Fig. 2). At 30 days, there was no significant difference in the primary outcome of treatment failure between the NCG and CG (16.7% vs. 18.8%; p= 0.65) (Fig. 2A). The secondary outcome of 30-day mortality occurred in eight and 11 patients in the NCG and the CG, respectively (14.8% vs. 17.2%; p= 0.63) (Fig. 2B). A Cox regression analysis showed no significant difference in the rate of treatment failure (HR, 1.32; 95% CI, 0.55-3.17; p= 0.54) (Fig. 3A) or 30-day mortality (HR, 1.75; 95% CI, 0.51-6.05; p= 0.38) (Fig. 3B) between the NCG and CG.
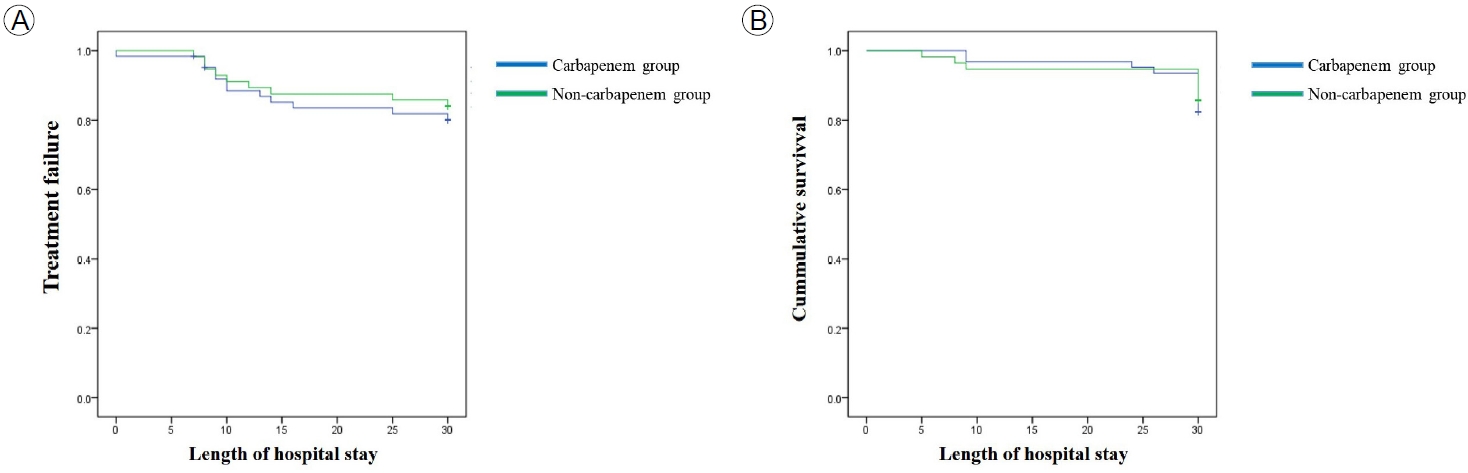
Survival of patients with extended-spectrum β-lactamase (ESBL)-Enterobacteriaceae bacteremia in the carbapenem and non-carbapenem groups by Kaplan-Meier analysis. (A) Treatment failure at 30 days, (B) 30-day mortality rate.
Risk factors for treatment failure of patients with ESBL-E bacteremia
In univariate analyses, we evaluated treatment failure according to age, sex, medical history, prior hospitalization, prior antimicrobial therapy, admission to the intensive care unit, PBS, type of antimicrobial therapy, PIP/TAZ minimum inhibition concentration (MIC) ≥ 32 μg/mL, extra-urinary tract infection, and species of pathogen (Table 2). Treatment failure differed significantly according to prior antimicrobial therapy within 30 days, extra-urinary tract infection, and Klebsiella pneumoniae. In a multivariate regression analysis, treatment failure differed significantly according to prior antimicrobial therapy within 30 days and extra-urinary tract infection. However, treatment failure did not show significant differences according to the other variables, including non-carbapenem antimicrobials.
DISCUSSION
The increasing incidence of ESBL-E is a challenge in healthcare, and effective treatment strategies are needed to mitigate the clinical and economic burden. We found a 30-day mortality rate of 16.1%, which is higher than the 10.3% in a 2020 South Korean study [6]. Despite this higher mortality rate, our findings challenge the prevailing preference for carbapenems, for ESBL-E bacteremia.
There was no significant difference in the treatment failure and 30-day mortality rates between the CG and NCG. Extra-urinary tract infection and prior antimicrobial therapy within 30 days were risk factors for treatment failure in patients with ESBL-E bacteremia. This contrasts with the outcomes of the MERINO trial, which compared PIP/TAZ with meropenem. In a randomized clinical trial involving 391 patients with bloodstream infections caused by ESBL-EC and Klebsiella pneumoniae, the primary outcome of 30-day mortality occurred in 12% and 4% of patients receiving PIP/TAZ and meropenem, respectively [12]. PIP/TAZ is not recommended for the treatment of infections other than those of the urinary tract, caused by ESBL-E, even if susceptibility to PIP/TAZ is demonstrated [7]. The choice of antimicrobial therapy is important in patient outcomes, and non-carbapenem alternatives should be considered. However, several design factors in the MERINO trial, such as the short duration of the intervention (median 6 days, compared to a median 13 days of treatment for bacteremia) and and overlap of drug exposure between groups, may have influenced the outcomes and generalizability [12]. Additionally, blaOXA-1 genes were highly prevalent (67%) and phenotypic ESBL production was confirmed in 86% of the isolates [12]. Testing for PIP/TAZ MIC may be inaccurate in the presence of other β-lactamases such as OXA-1, making it unclear if an isolate that tests susceptible to this agent is indeed susceptible. The high prevalence of blaOXA-1 genes suggests an explanation for the high failure rate of PIP/TAZ.
The population of this study was that of patients with ESBL-E bacteremia, whereas the MERINO trial focused on patients with ceftriaxone-resistant EC or Klebsiella spp. This could explain the differences in outcomes and the applicability of results to broader populations. Furthermore, our study highlights the importance of evaluating the characteristics of the individual patient when making treatment decisions. Despite a higher proportion of male patients in the CG, the clinical outcomes were similar in the two groups. This suggests that factors other than the antimicrobial used influence treatment efficacy and patient outcomes. The longer hospitalization duration in the CG raises questions about the cost-effectiveness and effect on patients of prolonged hospitalization. Optimizing hospital stays could improve patient outcomes and reduce healthcare costs, and so further research is needed. Extra-urinary tract infection and prior antimicrobial therapy within 30 days were risk factors for treatment failure in patients with ESBL-E bacteremia. Use of two or more antimicrobials, extended-infusion methods, and adequate drainage of the infection source should be considered for such patients. However, treatment failure did not differ significantly according to age group (≥ 80 years), sex, malignant cancer, prior hospitalization, nosocomial infection, non-carbapenem group, PIP/TAZ MIC ≥ 32 μg/mL, and strain of pathogen.
Our findings challenge the perceived effect of β-lactam/ β-lactamase inhibitors on susceptibility to antimicrobials. Based on data for patients with PIP/TAZ MICs ≤ 16 μg/mL, experts recommend carbapenem therapy for ESBL-E bloodstream infection (BSI) (11% PIP/TAZ vs. 4% carbapenems) and other common sites of infection [13]. In the NCG, PIP/TAZ administration according to MICs against PIP/TAZ demonstrated comparable efficacy in ESBL-E strains, suggesting that broad-spectrum carbapenems may not be necessary in all cases (HR, 1.40; 95% CI, 0.80-9.70 for MICs ≤ 16 μg/mL; HR, 1.90; 95% CI, 0.23-15.60 for MIC = 32 μg/mL; HR, 4.89; 95% CI, 0.38-30.50 for MICs ≥ 64 μg/mL). Although there were few cases with PIP/TAZ MICs ≥ 32 μg/mL (nine patients; median PBS, 0.0; interquartile range, 0.0-2.0), if the disease is not severe and the clinical course is stable, non-carbapenem drugs could be viable options. Furthermore, there was no significant association in the primary outcome among patients receiving antimicrobials other than PIP/TAZ compared to the CG (HR, 0.91; 95% CI, 0.31-2.60; p= 0.857).
We did not investigate the genetic characteristics of the isolates, which may have implications for understanding treatment responses and microbial-resistance patterns. In South Korea, between 2006 and 2011, the most common ESBL types were CTX-M-14 and CTX-M-15 (CTX-M-14, 41.7%; CTX-M-15, 35.0%; CTX-M-24, 8.7%; CTX-M-27, 7.8%; CTX-M-3, 3.9%; CTX-M-57, 2.9%) [14]. Between 2016 and 2017, CTX-M-15 and CTX-M-14 were the most common types [15]. Among the 249 cefotaxime-resistant isolates in a 2019 South Korean multicenter study, only CTX-M-14 and CTX-M-15 were identified [16]. Further molecular epidemiologic studies are needed to guide the management of community-onset infections caused by ESBL-E. Although the prevalence of ESBL enzymes such as OXA-1 have not increased in South Korea, continuous monitoring of other β-lactamases is important in the selection of non-carbapenem drugs.
An ongoing randomized controlled trial is evaluating PIP/TAZ for ESBL-E BSI. In an observational study, there was no difference in the 30-day mortality rate between treatment groups (94% vs. 97%, respectively) [8], and there was no significant difference in the 30-day mortality rate between PIP/TAZ and ertapenem for pyelonephritis or complicated urinary tract infection [17]. This finding mirrors the new IDSA guidelines, which recommend carbapenems for ESBL-E [9]. Antimicrobial drugs should be selected based on the resistance status of the pathogen, and the local antibiotic effectiveness profile.
This study has several limitations. First, it was a single-center retrospective study, and the small sample size could have reduced the statistical power. Second, PBS is used in infectious disease research as an index of the severity of acute disease. A PBS of ≥ 4 is used to define critical illness and an increased risk of mortality [18]. In this study, the disease severity was mild. Further research is needed to determine the effectiveness of non-carbapenem drugs in patients with severe disease. In addition, there were few cases with PIP/TAZ MICs ≥ 32 μg/mL. Moreover, the dynamic landscape of antimicrobial resistance necessitates ongoing evaluation of treatment strategies.
In conclusion, the findings suggest that non-carbapenem drugs can be alternatives for the treatment of ESBL-E bacteremia. This has implications for antimicrobial management and the fight against antibiotic resistance. Such an approach could reduce the selective pressure promoting carbapenem resistance and preserve the effectiveness of these crucial antibiotics. Further large prospective studies are needed to validate the results and refine treatment guidelines.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
This work was supported by the research grant from Jeju National University in 2023.
AUTHOR CONTRIBUTIONS
Conceptualization: Hyunnjoo Oh and Jeong Rae Yoo.
Data curation: Hyunnjoo Oh and Seunghee Lee.
Formal analysis: Hyunnjoo Oh and Seunghee Lee.
Funding acquisition: Jeong Rae Yoo.
Investigation: Misun Kim and Sang Taek Heo.
Methodology: Hyunnjoo Oh.
Project administration: Hyunnjoo Oh.
Resources: Hyunnjoo Oh.
Supervision: Jeong Rae Yoo.
Validation: Jeong Rae Yoo.
Visualization: Misun Kim and Sang Taek Heo.
Writing - original draft: Jeong Rae Yoo.
Writing - review & editing: all authors.
Approval of final manuscript: all authors.
Acknowledgements
We appreciate the data collection efforts of the Infection Control Unit team at Jeju National University Hospital.