 |
 |
| Korean J Med > Volume 99(2); 2024 > Article |
|
Abstract
Notes
AUTHOR CONTRIBUTIONS
Conception and design: YGL, DYK.
Acquisition of data: YGL.
Analysis and interpretation of data: YGL, HIG, SJK, HL, HN, SYH, DYK.
Drafting of the manuscript: YGL.
Critical revision for intellectual contents: YGL, HIG, SJK, HL, HN, SYH, DYK.
Final approval of the manuscript: YGL, HIG, SJK, HL, HN, SYH, DYK.
REFERENCES
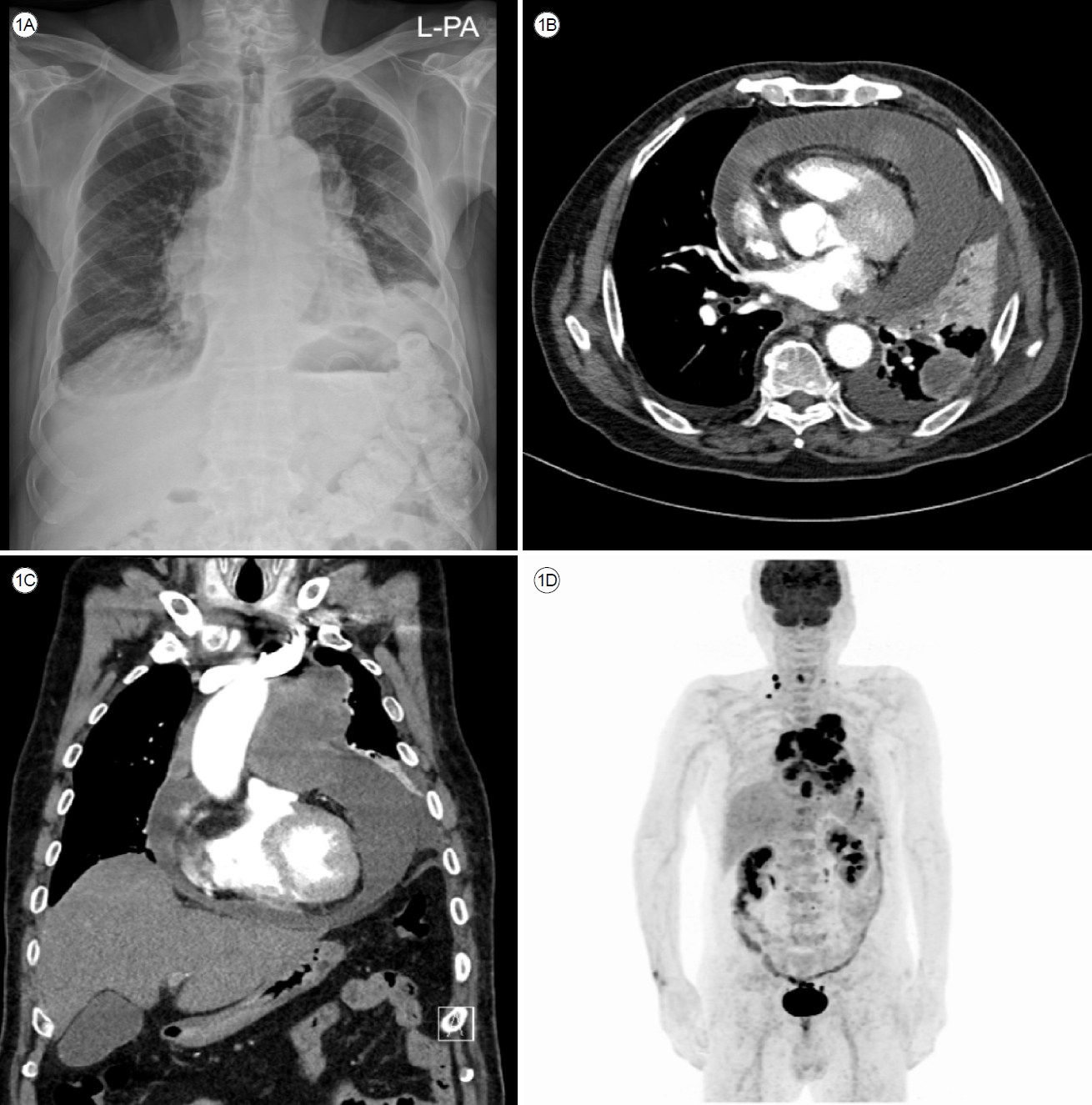
Figure┬Ā1.
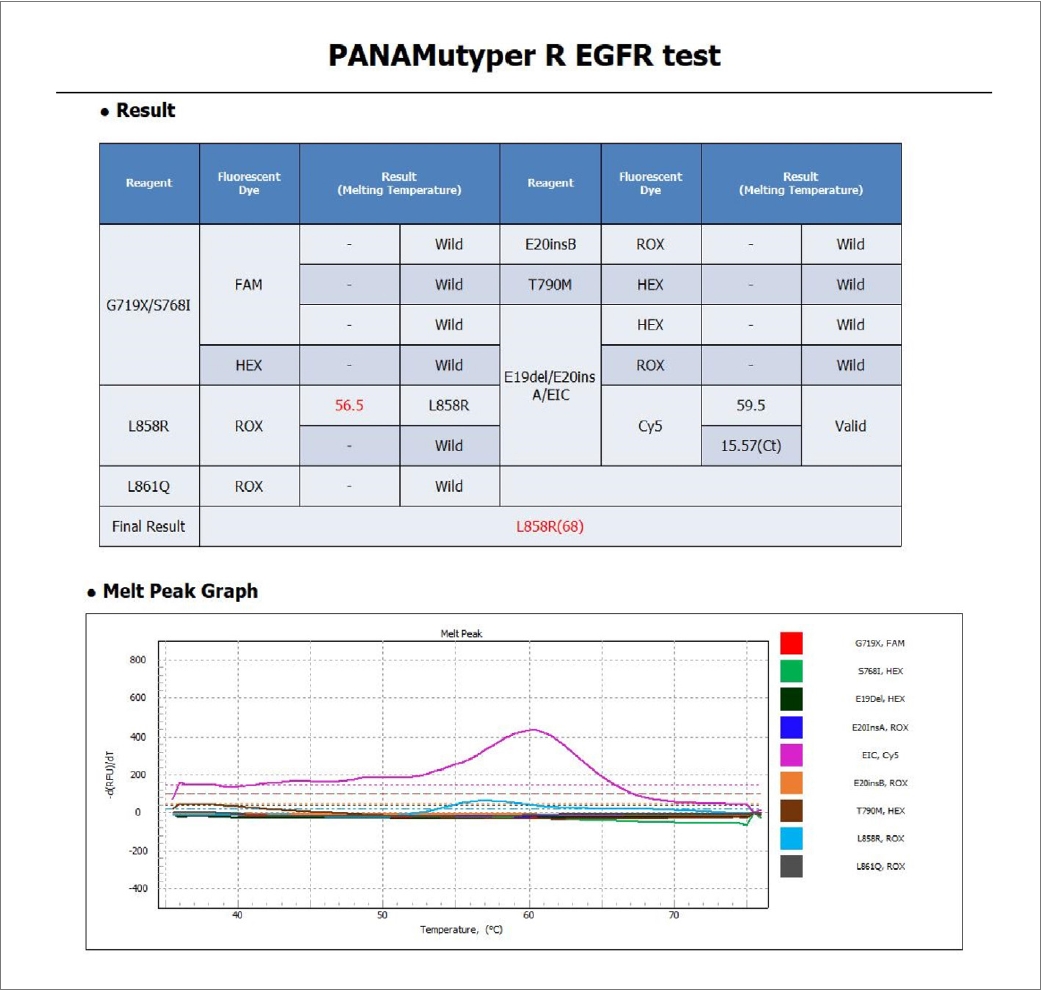
Figure┬Ā2.
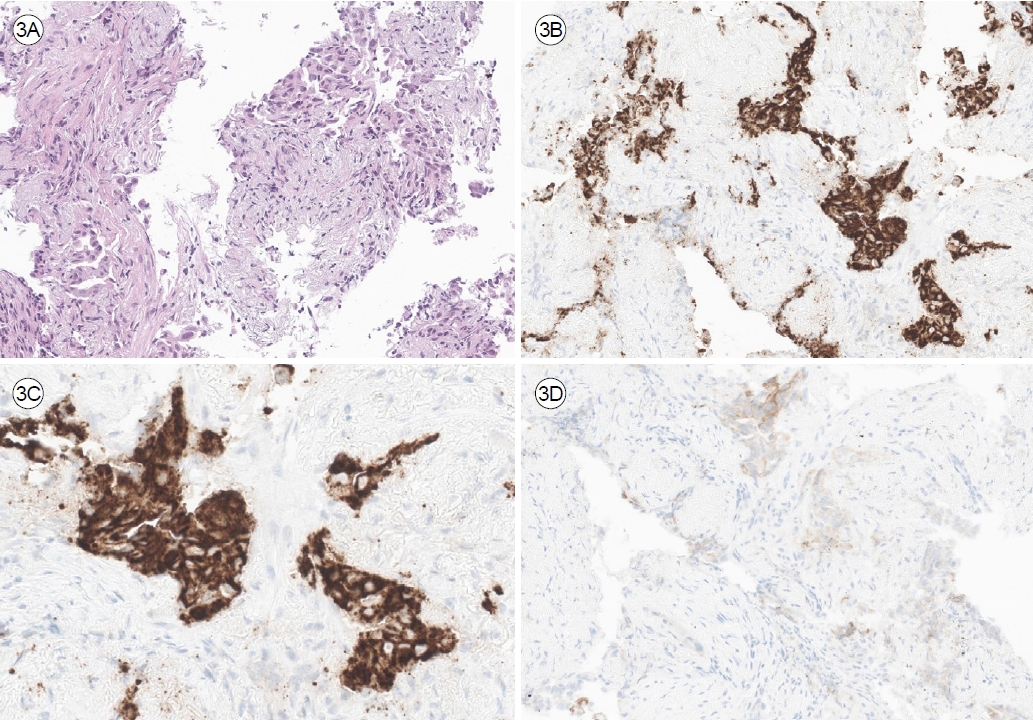
Figure┬Ā3.
Table┬Ā1.
| Biomarkers or genomic alterations | Methods of detection | Drug match |
|---|---|---|
| EGFR mutation | ||
| ŌĆāExon 19 deletion or exon 21 L858R | Sanger sequencing or NGS | EGFR TKI |
| ŌĆāEGFR T790M resistance mutation | Sanger sequencing or NGS | EGFR TKI |
| ŌĆāExon 19 deletion or exon 21 L858R except sensitizing EGFR mutation | Sanger sequencing or NGS | EGFR TKI |
| ŌĆāEGFR exon 20 insertion mutation | Sanger sequencing or NGS | EGFR TKI |
| ALK rearrangements | IHC | ALK TKI |
| FISH | ||
| RNA-based NGS | ||
| ROS1 rearrangements | IHCa | ROS1 TKI |
| FISH | ||
| RNA-based NGS | ||
| BRAF V600 mutation | Sanger sequencing or NGS | BRAF + MEK TKI |
| RET rearrangements | FISH | RET TKI |
| RNA-based NGSb | ||
| MET exon 14 skipping mutation | NGSc | MET TKI |
| MET gene amplification | IHC | MET TKI |
| ISH | ||
| HER2 mutation | NGS | Antibody-drug conjugate |
| KRAS G12C mutation | NGS | KRAS G12C inhibitor |
| NTRK rearrangement | IHCa | NTRK inhibitor |
| RNA-based NGS |
EGFR, epidermal growth factor receptor; NGS, next-generation sequencing; TKI, tyrosine kinase inhibitor; ALK, anaplastic lymphoma kinase; IHC, immunohistochemistry; FISH, fluorescence in situ hybridization; RNA, ribonucleic acid; ROS1, ROS proto-oncogene 1 receptor tyrosine kinase; MEK, mitogen-activated protein kinase kinase; RET, rearranged during transfection; MET, mesenchymal-epithelial transition; ISH, in situ hybridization; HER, human epidermal growth factor receptor; KRAS, Kristen rat sarcoma virus; NTRK, neurotrophic tyrosine receptor kinase.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print




