|
 |
| Korean J Med > Volume 99(2); 2024 > Article |
|
This article has been corrected. See "Human Understanding is Expected of the Physician: Proposing a Model of Disease Development" in Volume 100 on page 44.
Abstract
In HarrisonŌĆÖs Principles of Internal Medicine, human understanding is emphasized as one of three necessary characteristics that a physician must have. Inflammation, which is caused by inflammatory inducers (inf-ids), is a fundamental feature of disease at the cellular and molecular levels. Inflammation protects the body, but excessive or prolonged inflammation can be damaging and can cause disease. Humans are repeatedly exposed to external and internal environmental factors that generate inf-ids throughout their lives. External environmental factors include microbial and non-microbial inf-ids, as well as stressors that inevitably arise during social interactions. Internal environmental factors include the adaptive physiological response that is present from birth. Inf-ids may also be produced by the four-step habit loop, which consists of a cue (e.g., stressor), emotions, routine act (adaptive response), and a reward. Immune cells in the circulatory system and in tissues may have positive and negative effects in inflammatory responses. However, low-grade inflammation may be difficult to detect. We propose a model of disease development that integrates external and internal environmental factors from the perspective of human understanding.
Disease onset involves inflammation at the cellular and molecular levels [1-4]. Inflammation is mediated by the innate and acquired immune systems and it protects the body from infectious and non-infectious agents [1,2]. When the process progresses smoothly, inflammatory factors are removed by immune cells, and the damaged tissues are eventually repaired. However, if the inflammation is excessive or prolonged, cells and tissues can become damaged, resulting in various diseases [3,5,6]. Inflammation is an indispensable response that protects the human body, but it is also called the ŌĆśsilent killerŌĆÖ because it can provoke disease [4].
The first page of HarrisonŌĆÖs Principles of Internal Medicine states that physicians require technical skill, scientific knowledge, and human understanding [7,8]. In addition to human understanding, Shakespearean breadth has now been introduced, emphasizing the need for a broader understanding of human beings as exemplified in the works of the great English playwright William Shakespeare [8]. HarrisonŌĆÖs Principles of Internal Medicine suggests that to understand the ultimate source of a disease and provide effective treatment, it is necessary to have detailed insight into the stressors that the patient has experienced, is currently experiencing, or expects to experience in the future, as well as the emotions induced by those stressors. It is important to understand that disease involves more than simply damage to the components of the human body.
In a previous report, we suggested that a human understanding of patients and diseases from the perspective of Shakespearean breadth is necessary to treat pancreaticobiliary disease, reduce the rate of recurrence, and ultimately prevent the disease [9]. The biomedical basis of human understanding has been linked to the habit loop and the adaptive response that are unconsciously and automatically activated by stressors [10-14]. Based on a previously proposed model of human understanding that was applied to pancreaticobiliary disease [9], we suggest a model of disease development that includes inflammatory mechanisms at the cellular and molecular levels.
Diseases are conditions in which cells fail to maintain homeostasis due to molecular or structural alterations and damage caused by physical and chemical stimuli [2,15]. Various stimuli can damage cells and activate inflammatory pathways, which have four components: inducers, sensors, mediators, and target tissues (Fig. 1) [2,16-19]. Inducers stimulate the sensory receptors of immune cells, induce the secretion of inflammatory mediators (IMs), and may be classified as microbial or non-microbial [19]. Non-microbial inducers may be further classified as exogenous substances of inducers (Exo-SIs), which are introduced into the body from an external source, or endogenous substances of inducers (Endo-SIs), which are generated inside the body.
Exo-SIs generally enter the body through the nose, mouth, or skin [1]. Some Exo-SIs are harmful in any quantity, such as tobacco, air pollutants, asbestos fibers, crystalline silica, caustic substances, paraquat, coal tar, defoliants, perfluorooctanoic acid, polyethylene, and polyvinyl chloride [20-28]. Other Exo-SIs, including food and drink, may only be harmful if the body ingests more than it can process; this can result in excessive production of substances, such as cholesterol crystals, lipids, glucose, urate crystals, and acetaldehyde. Hyperlipidemia, hyperglycemia, hyperuricemia, or excessive production of aldehyde can stimulate monocytes, macrophages, endothelial cells, smooth muscle cells, mast cells, neutrophils, hepatocytes, Kupffer cells, and hepatic stellate cells, leading to the production of IMs [18,29-32]. In addition, high-fat and/or high-carbohydrate intake can elevate the levels of endotoxins, which are potent inflammatory antigens, in the blood [33-35].
Food may also be considered an Exo-SIs when consumed excessively. The lifestyles of many people have changed, and levels of physical activity have decreased due to the mechanization of labor and transport. Consequently, calorie intake is often excessive, and obesity has become more prevalent [36-39]. Obesity results in chronic low-grade inflammation (LGI) via the following mechanisms: dysregulated secretion of adipokines; secretion of cytokines and chemokines from macrophages, endothelial cells, and other immune cells in adipose tissue; cell death and hypoxia in adipose tissue; an increase in intestinal lipopolysaccharide-containing microbiota; and a decrease in the activity of the vagus nerve, which is a vital component of the inflammatory reflex [36,38-40].
Endo-SIs include stress hormones, pathogenic substances, physiological secretions (e.g., gastric acid, pancreatic juice, and bile), and metabolites (e.g., urine, stool, and sweat). Stress hormones are generated via the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system (SNS), which are activated by physical or psychological stressors [11,12,41,42]. When the HPA axis is activated, corticotropin-releasing factor is secreted from the hypothalamus, adrenocorticotropic hormone is released from the pituitary gland, and gucocorticoids (GCs) are secreted from the adrenal cortex. Stimulation of the somatic nervous system by stressors induces the secretion of norepinephrine (NE) from the terminal nerve and epinephrine (EP) from the adrenal medulla via the pons and spinal cord. NE, EP, and GCs bind to receptors on immune cells (e.g., neutrophils, monocytes, natural killer cells, macrophages, T cells, and B cells) in the blood, tissues, and organs (e.g., bone marrow, thymus, spleen, gut-associated lymphoid tissue, bronchus-associated lymphoid tissue, and lymph nodes); this activates intracellular signaling pathways and produces various IMs [3,11,13,43-45].
Pathogenic substances are associated with necrotic cell death and tumors. Three types of pathogenic substance are associated with necrotic cell death [46]. The first type includes chromatin-associated high-mobility group box 1 proteins, heat shock proteins, and purine metabolites (e.g., adenosine triphosphate and uric acid); these are released from cells after the plasma membranes are ruptured. The second type includes hyaluronan, heparan sulfate, and biglycan, which are produced after the extracellular matrix is degraded. The third type includes the biologically active pro-inflammatory cytokines and chemokines present in cells, including interleukin (IL)-1a and IL-33.
Pathogenic substances are also secreted by tumors or induced by cancer therapy. The tumor microenvironment consists of inflammatory cells that stimulate tumor proliferation and migration [47-50]. Tumor cells secrete cytokines and chemokines to attract leukocytes, such as neutrophils, dendritic cells, macrophages, eosinophils, mast cells, and lymphocytes. These leukocytes produce cytokines and cytotoxic mediators to further promote inflammation. Cancer therapies include chemotherapy, radiotherapy, and immunotherapy; these treatments kill tumor cells, releasing substances that stimulate inflammation [51,52].
The other Endo-SIs include enzymes (e.g., in saliva, gastric acid, pancreatic juice, and bile) and metabolites (e.g., in urine, stool, and sweat) that are involved in normal physiological and metabolic processes. These play roles in digestion and maintain balance in the internal environment [53]. However, there are two situations in which these can become Endo-SIs and cause inflammation. This may occur if the site of action is altered. For example, gastric acid and pepsin facilitate the digestion of food but can cause inflammation in the esophageal epithelium if they are refluxed into the esophagus [54,55]. In the congenital anomaly of pancreaticobiliary maljunction, digestive pancreatic juice is released into the biliary tract causing inflammation [56]. Inflammation can also occur if gastric acid, bile, pancreatic juice, or stool is released into the abdominal, retroperitoneal, or thoracic cavities following gastroduodenal or intestinal perforation or pancreatitis [57,58]. The other situation in which enzymes or metabolites can become Endo-SIs is when their excretion is impaired. Bile accumulates in the biliary tract or blood when it cannot be secreted into the duodenum due to hepatitis, gallbladder dyskinesia, sphincter of Oddi dysfunction, or a biliary obstruction. This may cause cholecystitis, hepatitis, neurotoxicity, or systemic inflammatory response syndrome [59-61]. Furthermore, various products of protein metabolism are excreted in the urine. If renal failure inhibits the excretion of these products, they accumulate in the blood and become inflammatory inducers (inf-ids) that stimulate systemic inflammation [62,63].
People are exposed to Exo-SIs and microbial inducers throughout their lifetimes [64]. They frequently eat, drink, and inhale Exo-SIs (e.g., food, cigarettes, alcohol, and air pollutants) for survival or pleasure, or even unconsciously. In particular, humans have an inherent fear of starvation and tend to overeat unknowingly [65]. In addition, microbes are always present both inside and outside the human body [1].
Psychological stress can produce Endo-SIs at various times of life [66-69]. As seen in the many plays written by William Shakespeare, stress results from conflicts when people with different backgrounds, cognitive biases, values, and goals interact. Moreover, stressors are natural and inevitable in human life. Humans have evolved the adaptive response to situations in which survival or self-esteem is threatened [11,12,70,71]. This adaptive response includes a physical (behavioral) reaction that activates the skeletal muscles and a physiological reaction that activates the internal organs [11,72,73]. These reactions are stimulated by emotions that occur when a particularly stressful situation is encountered [11,13,70,72-74]. Cannon reported that the physical fight-or-flight response is activated by anger or fear, with fighting being a response to anger and flight a response to fear [70]. Among the stress hormones that can function as Endo-SIs (EP, NE, and GCs), catecholamine (EP and NE) plays a key role in the fight-or-flight response [11,45,70,75]. Interestingly, the HPA axis and SNS are always activated simultaneously and produce stress hormones [11,41-43,45]. Thus, anger and fear stimulate the fight-or-flight response and the physiological reaction. Likewise, other emotions, such as despair, depression, sadness, or anxiety can also trigger the adaptive response [11-13,69].
Emotions are often responsible for human behaviors [76-79]. Emotions also synchronize those behaviors that introduce Exo-SIs into the human body, such as overeating, alcohol consumption, and smoking [80]. In addition, emotions may affect food choices and eating patterns. Strong emotions may inhibit food intake, but less intense emotions may have unpredictable effects on eating habits [81,82]. Sometimes, sweets and high-calorie foods are consumed in response to negative emotions. One study reported a 32% higher prevalence of depression among people who were overweight or obese compared to people of normal weight [83].
Smoking and excessive alcohol consumption may sometimes calm negative emotions [84-86]. According to a segmentation study in 1981, smoking may relieve anxiety, depressive symptoms, and stress; it may also improve self-control [87]. The tension reduction theory suggests that alcohol consumption affects the central nervous system and reduces stress [88].
Interestingly, the adaptive response is also activated by perceived threats [11,13,44,89,90]. Therefore, regardless of authenticity, if a situation is perceived as threatening, the adaptive response is triggered automatically. Furthermore, even in a genuinely threatening situation, the adaptive response may vary depending on the appraisal of the situation [11,75,89]. In fact, the adaptive response occurs only when the individual perceives and appraises a situation as a threat to survival. Finally, the adaptive response can be triggered by recollection of past events or anxiety about the future [11,12,79,91-93].
Past experiences that are associated with pain or fear, such as childhood trauma, sexual assault, war, traffic accidents, or life-threatening situations can cause schizokinesis and may be stored in long-term memory [94-96]. As demonstrated by PavlovŌĆÖs conditioned reflex, the recall of invisible memories can activate a visible physiological response (e.g., saliva) [97]. Traumatic experiences in childhood can alter stress sensitivity, triggering the adaptive response under relatively low levels of stress [91,98-103]. Traumatic childhood experiences can also result in chronic activation of the adaptive response for decades, repeatedly generating Endo-SIs [102,104]. In addition, very severe childhood trauma may increase the frequency of Exo-SIs production and exacerbate problems (e.g., with alcohol consumption, smoking, physical inactivity, drug abuse, or obesity) [99,105].
Therefore, systems that are designed to maintain homeostasis can damage the human body and cause disease [2,4,11,12]. As described by Cannon, emotions have evolved to trigger and control the fight-or-flight response, which is essential for survival [70,106]. Such emotions can also maintain homeostasis and they are key components in the four-step habit loop for coping with stressors [9,77,107]. The adaptive response operates automatically in life-threatening situations [11,12,75]. Immune cells play pivotal roles in eliminating pathogenic inducers [4,108,109]. These internal systems protect the human body from stressors, Exo-SIs, and Endo-SIs. However, inappropriate, excessive, or prolonged activation of these systems can damage the human body [2-6,11,12,17,19,107-109].
Inf-ids may be repeatedly introduced from outside the human body or produced inside the body due to habit loops that are initiated by stressors [9,10,14,110]. Habit loops are behavioral patterns that operate reflexively in response to similar situations. When emotions are triggered by stressors, the adaptive response may be activated immediately. Consequently, Exo-SIs are introduced into the body using the skeletal muscles as an alternative behavioral pattern to the fight-or-flight response; the physiological response may also be activated by stress hormones. Subsequently, emotional tension is temporarily relieved, and there is a psychological reward. Although this psychological compensation is temporary, individuals will tend to routinely repeat the same behavioral pattern when they encounter similar situations to achieve psychological relief [9,10,14,110]. Therefore, familiar stressors create a four-step habit loop involving a cue (e.g., stressor), emotions, routine act (adaptive response), and a reward [9].
A chronic protective inflammatory response can result in disease. This may occur when inf-ids persist and the inflammatory response is lower than normal [1,2,111,112]. Pathogenesis can also develop if the link between the function of the systemic anti-inflammatory response and the diffusible neural network that controls the spread of inflammation is disrupted [111,112]. Changes in the pattern of disease can also lead to persistent inflammation. In the past, many diseases were caused by acute inflammation due to infections or injuries. However, most chronic inflammatory diseases are now due to excessive food or alcohol consumption, tobacco, air pollutants, or psychosocial stress [2,15,16]. A key characteristic of chronic inflammatory diseases is the presence of LGI, in which typical inflammatory symptoms (e.g., pain, fever, erythema, and swelling) are not evident [2]. LGI involves only a two- to four-fold increase in the levels of inflammatory markers such as cytokines and acute phase proteins [113-115]. LGI differs from that observed in typical acute inflammation because the level of inflammation is relatively low and it is due to other than an infection or tissue injury. In addition, the inflammation is systemic rather than localized and occurs over a longer period [16]. Pain, warmth, swelling, and erythema can be used to recognize and treat acute inflammation but LGI is more difficult to detect. However, if LGI persists, it may result in damage to cells and loss of organ function. LGI is characteristic of chronic diseases and is often associated with aging, hypertension, coronary heart disease, type 2 diabetes, metabolic syndrome, chronic obstructive pulmonary disease, osteoarthritis, post-traumatic stress disorder, depression, autoimmune diseases, functional dyspepsia, atriall fibrillation, and cancer [5,64,113-133].
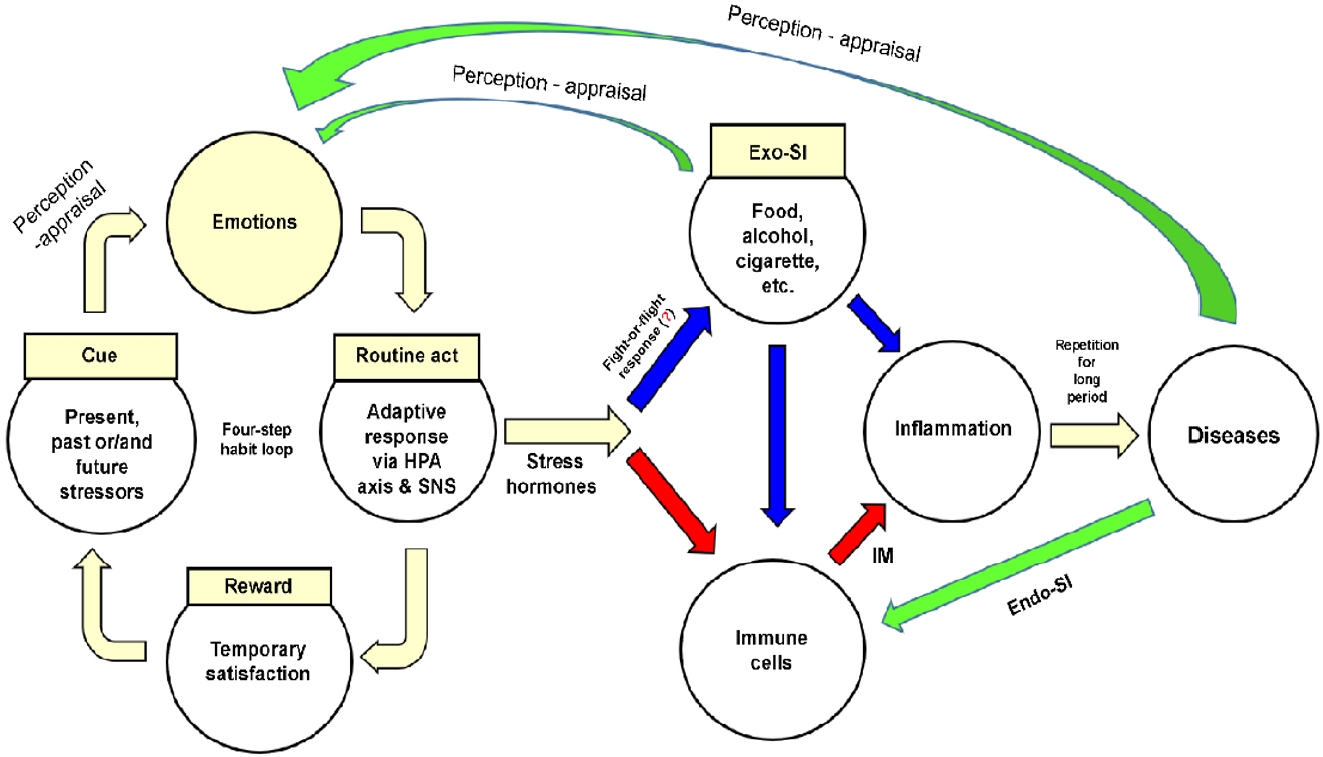
Figure 2 shows a model of disease development that integrates human understanding and involves external environmental and internal human characteristics, as described in the previous section. Stressors formed by memories, present situations, or future concerns activate the four-step habit loop. Emotions are elicited after the stressor is perceived and routine act (adaptive response) is triggered. The emotional tension is temporarily relieved, and a psychological reward is obtained. Individuals routinely repeat the four-step habit loop whenever they encounter similar stressors.
The adaptive response of the four-step habit loop immediately activates the HPA axis and SNS, having two effects. First, the skeletal muscles are stimulated to introduce Exo-SIs (e.g., food, alcohol, or tobacco) into the body. However, excessive introduction of Exo-SIs may cause inflammation of the target tissue or produce IMs by stimulating immune cells in the blood or tissues. Second, a physiological reaction (involving GCs from the HPA axis or NE and EP from the SNS and adrenal medulla) produces IMs by stimulating immune cells. The IMs together with recruited immune cells cause inflammation in the target tissues. When inflammation is repeatedly triggered by Exo-SIs and/or Endo-SIs over a prolonged period, the target tissues are unable to recover and cells become irreversibly damaged. Eventually, organ function is impaired and disease develops.
Chronic inflammation becomes part of a vicious cycle before and after disease development. First, emotions elicited when Exo-SIs are introduced into the body reactivate the four-step habit loop [134]. Second, the presence of disease is perceived as a threat, inducing negative emotions such as despair, fear, and anger [69,135], this also activates the four-step habit loop. Third, tissue necrosis due to the disease, substances secreted by tumor cells (e.g., cytokines and chemokines), and substances that have accumulated due to the disease (e.g., bile, pancreatic juice, urine, and urea) stimulate immune cells and exacerbate inflammation.
This model has some limitations. First, the model is applicable only to diseases caused by repeated overstimulation of inf-ids over a prolonged period and may not apply to diseases caused by nutrient deficiencies. Second, the model may be less applicable to diseases that do not involve the four-step habit loop. Although diseases caused by congenital chromosomal abnormalities are due to an excess or deficiency of critical substances, they are not caused by stressors and emotions but rather by chromosomal alterations. Similarly, the model may not be applicable to diseases caused by infections because here the relationship between disease and the four-step habit loop is weak except infections associated with compulsive sexual behavior and injection drug use [136-139]. Third, the model is not suitable for understanding the impact of involuntary Exo-SIs, such as fine-particulate air and water pollutants produced in the individualŌĆÖs occupational environment or by industry or traffic (e.g., lead, diesel, tire particles, asbestos fibers, crystalline silica, defoliants, perfluorooctanoic acid, polyethylene, and polyvinyl chloride). Fourth, even when stressors, Exo-SIs, and/or Endo-SIs are similar, not all individuals will develop the disease, or the same disease even if they do. Such a phenomenon is also difficult to explain with this model. The incidence of disease may vary depending on individual differences, such as in the ability to perceive stressors, physical health, muscle volume, telomere length, dehydration, and microbiota [11,140-144]. For example, the same stressors may elicit different negative emotions in different individuals [11,69,145]. Essential factors of the pathogenesis of diseases usually include environmental and genetic factors [65,73,146]. In addition, personal factors, such as perception and interpretation abilities, emotion control, and/or the four-step habit loop may have an important impact on pathogenesis [11,69,75,96,145,147]. Fifth, chronic disease development is frequently associated with LGI [113-133]. However, recognizing LGI is difficult because the signs associated with acute inflammation are absent or difficult to detect. Our model does not explain why the cellular damage that leads to disease cannot be detected early, and further studies on this topic are needed. Furthermore, diagnostic markers and tools that can detect LGI, such as the high-sensitivity C-reactive protein test, are required [148,149].
The requirement for human understanding means that physicians need an awareness of the external environment, as well as internal structural and functional characteristics, to monitor disease progression and provide effective treatments [9,69,91,96]. The human body is not sufficiently robust to withstand ongoing inflammation over a prolonged period, and individuals are constantly exposed to microbial and non-microbial inducers that can cause inflammation throughout their lives. Individuals have both adaptive and innate immune systems from birth, whereas the four-step habit loop and acquired immune system develop gradually thereafter. These function as protective systems that maintain homeostasis and promote survival against a background of changes in the external and internal environments. However, paradoxically, these systems may also damage the human body if they do not develop correctly or are activated for a prolonged period. They may be activated unconsciously in response to current situations but can also be triggered by recollection of past events or anxiety about the future. Importantly, the LGI that is frequently associated with chronic disease is difficult to detect.
Diseases are not simply disorders of the structural components of the human body but are also influenced by stressors experienced in the past or present, or anticipated in the future. Diseases may result from the interaction between genetic and environmental factors. Furthermore, how an individual perceives and interprets stressors, controls his or her emotions, and adjusts his or her four-step habit loop may be crucial.
The priority in treatment is recovery from the ill health caused by stimulation with microbial or non-microbial inducers. From the perspective of human understanding, treatment may be enhanced by increasing the patientŌĆÖs awareness of the four-step habit loop because this mediates the repeated ingestion of Exo-SIs and/or produces Endo-SIs in response to stressors. Treatments should also be designed to reduce the likelihood of disease recurrence. Furthermore, disease development may be prevented by understanding how stress results from conflict when people with different values and goals interact, similar to the characters described in the many plays written by William Shakespeare. In conclusion, a disease development model that embraces the internal and external environmental characteristics described above is needed to prevent and treat disease. Therefore, we present a model of disease development that integrates human understanding and involves these characteristics.
Notes
AUTHOR CONTRIBUTIONS
Conceptualization: S.H.P; Methodology: H.A.L; Software: S.P, J.Y.K; Validation: S.W.C, S.B.B, T.H.L, J.H.M; Investigation: S.H.P, H.A.L; Resources: S.H.P, S.H.J; Writing - original draft preparation: S.H.P, S.P, J.Y.K; Writing - review and editing: J.Y.K, S.P, S.H.P; Visualization: S.M.L; Supervision: S.H.P; Project administration: S.H.P.
Acknowledgements
The authors would like to thank Shi Nae Yu, M.D., Division of Infectious Diseases, Department of Internal Medicine, Sae Hwan Lee, M.D., Division of Gastroenterology, Department of Internal Medicine, Young Sin Cho, M.D., Division of Gastroenterology, Department of Internal Medicine, and Jin Myoung Seok, M.D., Department of Neurology, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea, for recommending references.
REFERENCES
3. Tian R, Hou G, Li D, Yuan TF. A possible change process of inflammatory cytokines in the prolonged chronic stress and its ultimate implications for health. ScientificWorldJournal 2014;2014:780616.



4. Kumar V, Abbas AK, Aster JC. Inflammation and repair. In: Kumar V, Abbas AK, Aster JC, eds. Robbins & Cotran pathologic basis of disease. 10th ed. Philadelphia: Elsevier, 2021:71-113
6. Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res 2009;15:425ŌĆō430.


7. Loscalzo J, Fauci A, Kasper D, Hauser S, Longo D, Jameson JL. The practice of medicine. In: Loscalzo J, Fauci A, Kasper D, Hauser S, Longo D, Jameson JL, eds. Harrison's principles of internal medicine. 21st ed. Vol. 1. New York: McGraw-Hill, 2022:1-8
8. Fauci AS, Braunwald E, Isselbacher KJ, et al. The practice of medicine. In: Fauci AS, Braunwald E, Isselbacher KJ, et al., eds. Harrison's principles of internal medicine. 14th ed. Vol. 1. New York: McGraw Hill, 1998:1-6
9. Park SH, Song HR, Lee SW, Lee TH. Wouldn't human understanding be necessary to treat patients with pancreaticobiliary disease more effectively? Korean J Pancreas Biliary Tract 2018;23:89ŌĆō100.

10. Duhigg C. The power of habit: why we do what we do in life and business. New York: Penguin Random House, 2012.
11. McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med 1998;338:171ŌĆō179.


12. Gonz├Īlez-D├Łaz SN, Arias-Cruz A, Elizondo-Villarreal B, Monge-Ortega OP. Psychoneuroimmunoendocrinology: clinical implications. World Allergy Organ J 2017;10:19.



13. Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol 2005;5:243ŌĆō251.


14. Clear J. Atomic habits: an easy & proven way to build good habits & break bad ones. New York: Avery Publishing Group, 2018.
15. Kotas ME, Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell 2015;160:816ŌĆō827.



16. Rohleder N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom Med 2014;76:181ŌĆō189.


17. Marx J. Cancer research. Inflammation and cancer: the link grows stronger. Science 2004;306:966ŌĆō968.


18. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685ŌĆō1895.

20. McCusker K. Mechanisms of respiratory tissue injury from cigarette smoking. Am J Med 1992;93:18SŌĆō21S.

21. Pope CA 3rd, Ezzati M, Dockery DW. Fine-particulate air pollution and life expectancy in the United States. N Engl J Med 2009;360:376ŌĆō386.



22. Steenland K, Stayner L. Silica, asbestos, man-made mineral fibers, and cancer. Cancer Causes Control 1997;8:491ŌĆō503.


23. Fujiki H. Gist of Dr. Katsusaburo Yamagiwa's papers entitled "Experimental study on the pathogenesis of epithelial tumors" (I to VI reports). Cancer Sci 2014;105:143ŌĆō149.


24. Seok SJ, Gil HW, Jeong DS, Yang JO, Lee EY, Hong SY. Paraquat intoxication in subjects who attempt suicide: why they chose paraquat. Korean J Intern Med 2009;24:247ŌĆō251.



25. Steenland K, Fletcher T, Savitz DA. Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA). Environ Health Perspect 2010;118:1100ŌĆō1108.



26. Hoffman RS, Burns MM, Gosselin S. Ingestion of caustic substances. N Engl J Med 2020;382:1739ŌĆō1748.


27. Kang HK, Dalager NA, Needham LL, et al. Health status of Army Chemical Corps Vietnam veterans who sprayed defoliant in Vietnam. Am J Ind Med 2006;49:875ŌĆō884.


28. Marfella R, Prattichizzo F, Sardu C, et al. Microplastics and nanoplastics in atheromas and cardiovascular events. N Engl J Med 2024;390:900ŌĆō910.


29. de Vries MA, Klop B, Janssen HW, Njo TL, Westerman EM, Cabezas MC. Postprandial inflammation: targeting glucose and lipids. Adv Exp Med Biol 2014;824:161ŌĆō170.

30. Perez-Ruiz F, Castillo E, Chinchilla SP, Herrero-Beites AM. Clinical manifestations and diagnosis of gout. Rheum Dis Clin North Am 2014;40:193ŌĆō206.


31. Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 2011;141:1572ŌĆō1585.



33. Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr 2007;86:1286ŌĆō1292.

34. Ghanim H, Abuaysheh S, Sia CL, et al. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care 2009;32:2281ŌĆō2287.
35. Laugerette F, Vors C, G├®lo├½n A, et al. Emulsified lipids increase endotoxemia: possible role in early postprandial lowgrade inflammation. J Nutr Biochem 2011;22:53ŌĆō59.


36. Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol 2011;29:415ŌĆō445.


37. Drake I, Sonestedt E, Ericson U, Wallstr├Čm P, Orho-Melander M. A Western dietary pattern is prospectively associated with cardio-metabolic traits and incidence of the metabolic syndrome. Br J Nutr 2018;119:1168ŌĆō1176.


38. Balistreri CR, Caruso C, Candore G. The role of adipose tissue and adipokines in obesity-related inflammatory diseases. Mediators Inflamm 2010;2010:802078.



40. Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex--linking immunity and metabolism. Nat Rev Endocrinol 2012;8:743ŌĆō754.



41. Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med 1995;332:1351ŌĆō1362.


42. Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol 2004;5:617ŌĆō625.


43. Downing JE, Miyan JA. Neural immunoregulation: emerging roles for nerves in immune homeostasis and disease. Immunol Today 2000;21:281ŌĆō289.


44. Black PH. The inflammatory response is an integral part of the stress response: implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav Immun 2003;17:350ŌĆō364.


45. Oberbeck R. Catecholamines: physiological immunomodulators during health and illness. Curr Med Chem 2006;13:1979ŌĆō1989.


46. Chen GY, Nu├▒ez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 2010;10:826ŌĆō837.



47. Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 2009;30:1073ŌĆō1081.


50. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008;454:436ŌĆō444.


51. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity 2019;51:27ŌĆō41.

52. de Freitas Saito R, Rangel MC, Chandler M, Beasock D, Afonin KA, Chammas R. Cancer therapy-induced inflammation and its consequences. In: de Araujo DR, Carneiro-Ramos M, eds. Biotechnology applied to inflammatory diseases: cellular mechanisms and nanomedicine. Berlin: Springer, 2023:49-75
53. Hall JE, Hall ME. Guyton and Hall textbook of medical physiology. 14th. Amsterdam: Elsevier, 2020.
54. Barbera M, Fitzgerald RC. Cellular mechanisms of Barrett's esophagus development. Surg Oncol Clin N Am 2009;18:393ŌĆō410.


55. Vaezi MF, Richter JE. Role of acid and duodenogastroesophageal reflux in gastroesophageal reflux disease. Gastroenterology 1996;111:1192ŌĆō1199.

56. Kamisawa T, Takuma K, Anjiki H, et al. Pancreaticobiliary maljunction. Clin Gastroenterol Hepatol 2009;7 Suppl 11:S84ŌĆōS88.


57. Langell JT, Mulvihill SJ. Gastrointestinal perforation and the acute abdomen. Med Clin North Am 2008;92:599ŌĆō625, viii-ix.


58. S├Ėreide K, Thorsen K, Harrison EM, et al. Perforated peptic ulcer. Lancet 2015;386:1288ŌĆō1298.



59. Sherman S, Lehman GA. Sphincter of Oddi dysfunction: diagnosis and treatment. JOP 2001;2:382ŌĆō400.

60. Brites D. The evolving landscape of neurotoxicity by unconjugated bilirubin: role of glial cells and inflammation. Front Pharmacol 2012;3:88.



61. Pavlidis ET, Pavlidis TE. Pathophysiological consequences of obstructive jaundice and perioperative management. Hepatobiliary Pancreat Dis Int 2018;17:17ŌĆō21.


62. Guarnieri G, Biolo G, Zanetti M, Barazzoni R. Chronic systemic inflammation in uremia: potential therapeutic approaches. Semin Nephrol 2004;24:441ŌĆō445.


63. Cantarin MPM, Whitaker-Menezes D, Lin Z, Falkner B. Uremia induces adipose tissue inflammation and muscle mitochondrial dysfunction. Nephrol Dial Transplant 2017;32:943ŌĆō951.


64. Franceschi C, Capri M, Monti D, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 2007;128:92ŌĆō105.


65. Goldman L. Too much of a good thing: how four key survival traits are now killing us. New York: Little, Brown Spark, 2015.
66. Kim YK, Maes M. The role of the cytokine network in psychological stress. Acta Neuropsychiatr 2003;15:148ŌĆō155.


67. Sorrells SF, Caso JR, Munhoz CD, Sapolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron 2009;64:33ŌĆō39.



68. Maniam J, Morris MJ. The link between stress and feeding behaviour. Neuropharmacology 2012;63:97ŌĆō110.


69. Simonton OC, Creighton J, Simonton SM, Matthews S, Creighton JL. Getting well again: the bestselling classic about the Simontons' revolutionary lifesaving self-awareness techniques. New York: Bantam Books, 1992.
70. Cannon WB. The emergency function of the adrenal medulla in pain and the major emotions. Am J Physiol 1914;33:356ŌĆō372.

71. Varki A. Nothing in medicine makes sense, except in the light of evolution. J Mol Med (Berl) 2012;90:481ŌĆō494.


72. Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull 2004;130:601ŌĆō630.



73. Mawdsley JE, Rampton DS. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut 2005;54:1481ŌĆō1491.



75. Szabo S, Tache Y, Somogyi A. The legacy of Hans Selye and the origins of stress research: a retrospective 75 years after his landmark brief "letter" to the editor# of Nature. Stress 2012;15:472ŌĆō478.


76. Ekman P. Emotions revealed: recognizing faces and feelings to improve communication and emotional life. 2nd. New York: St. Martin's Press, 2007.
77. Zeelenberg M, Nelissen RMA, Breugelmans SM, Pieters R. On emotion specificity in decision making: why feeling is for doing. Judgm Decis Mak 2008;3:18ŌĆō27.

78. Phelps EA, Lempert KM, Sokol-Hessner P. Emotion and decision making: multiple modulatory neural circuits. Annu Rev Neurosci 2014;37:263ŌĆō287.


79. Lerner JS, Li Y, Valdesolo P, Kassam KS. Emotion and decision making. Annu Rev Psychol 2015;66:799ŌĆō823.

80. Ulrich-Lai YM, Fulton S, Wilson M, Petrovich G, Rinaman L. Stress exposure, food intake and emotional state. Stress 2015;18:381ŌĆō399.


82. Evers C, Dingemans A, Junghans AF, Boev├® A. Feeling bad or feeling good, does emotion affect your consumption of food? A meta-analysis of the experimental evidence. Neurosci Biobehav Rev 2018;92:195ŌĆō208.


83. Pereira-Miranda E, Costa PRF, Queiroz VAO, Pereira-Santos M, Santana MLP. Overweight and obesity associated with higher depression prevalence in adults: a systematic review and meta-analysis. J Am Coll Nutr 2017;36:223ŌĆō233.


84. Wills TA. Stress and coping in early adolescence: relationships to substance use in urban school samples. Health Psychol 1986;5:503ŌĆō529.


85. Leventhal H, Cleary PD. The smoking problem: a review of the research and theory in behavioral risk modification. Psychol Bull 1980;88:370ŌĆō405.


86. Russell JA, Mehrabian A. The mediating role of emotions in alcohol use. J Stud Alcohol 1975;36:1508ŌĆō1536.


87. Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. JAMA 2000;284:2606ŌĆō2610.


88. Sayette MA. The effects of alcohol on emotion in social drinkers. Behav Res Ther 2017;88:76ŌĆō89.



89. Black PH, Garbutt LD. Stress, inflammation and cardiovascular disease. J Psychosom Res 2002;52:1ŌĆō23.


90. Szalay J. What is inflammation [Internet]. New York (NY): Live Science, c2018 [cited 2018 Oct 19]. Available from: http://www.livescience.com/52344-inflammation.html
91. Harris NB. The deepest well: healing the long-term effects of childhood adversity. Boston: Houghton Mifflin Harcourt, 2018.
92. Schulkin J, McEwen BS, Gold PW. Allostasis, amygdala, and anticipatory angst. Neurosci Biobehav Rev 1994;18:385ŌĆō396.


93. Schulz P, Kirschbaum C, Pr├╝├¤ner J, Hellhammer D. Increased free cortisol secretion after awakening in chronically stressed individuals due to work overload. Stress Med 1998;14:91ŌĆō97.

94. Gantt WH. Principles of nervous breakdown-schizokinesis and autokinesis. Ann N Y Acad Sci 1953;56:143ŌĆō163.


95. Kandel ER. In search of memory: the emergence of a new science of mind. 1st ed. New York: W. W. Norton & Company, 2007.
96. Lown B. The lost art of healing: practicing compassion in medicine. New York: Ballantine Books, 1999.
97. Pavlov PI. Conditioned reflexes: an investigation of the physiological activity of the cerebral cortex. Ann Neurosci 2010;17:136ŌĆō141.



98. Chaplin TM, Niehaus C, Gon├¦alves SF. Stress reactivity and the developmental psychopathology of adolescent substance use. Neurobiol Stress 2018;9:133ŌĆō139.



99. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. Am J Prev Med 1998;14:245ŌĆō258.

100. National Scientific Council. Excessive stress disrupts the development of brain architecture. J Child Serv 2014;9:143ŌĆō153.

101. Teicher MH, Samson JA. Annual research review: enduring neurobiological effects of childhood abuse and neglect. J Child Psychol Psychiatry 2016;57:241ŌĆō266.



102. Koss KJ, Gunnar MR. Annual research review: early adversity, the hypothalamic-pituitary-adrenocortical axis, and child psychopathology. J Child Psychol Psychiatry 2018;59:327ŌĆō346.



103. Grasso DJ, Ford JD, Briggs-Gowan MJ. Early life trauma exposure and stress sensitivity in young children. J Pediatr Psychol 2013;38:94ŌĆō103.



104. Danese A, Lewis SJ. Psychoneuroimmunology of early-life stress: the hidden wounds of childhood trauma? Neuropsychopharmacology 2017;42:99ŌĆō114.



105. Schwandt ML, Heilig M, Hommer DW, George DT, Ramchandani VA. Childhood trauma exposure and alcohol dependence severity in adulthood: mediation by emotional abuse severity and neuroticism. Alcohol Clin Exp Res 2013;37:984ŌĆō992.



107. Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 1992;267:1244ŌĆō1252.


108. Wright HL, Moots RJ, Bucknall RC, Edwards SW. Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford) 2010;49:1618ŌĆō1631.


109. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012;122:787ŌĆō795.

110. Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 2010;68:815ŌĆō834.



112. Andersson U, Tracey KJ. Neural reflexes in inflammation and immunity. J Exp Med 2012;209:1057ŌĆō1068.

113. Br├╝├╝nsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am 2003;23:15ŌĆō39.


114. Tkacova R. Systemic inflammation in chronic obstructive pulmonary disease: may adipose tissue play a role? Review of the literature and future perspectives. Mediators Inflamm 2010;2010:585989.



115. Dinh QN, Drummond GR, Sobey CG, Chrissobolis S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res Int 2014;2014:406960.



116. Danesh J. Smoldering arteries? Low-grade inflammation and coronary heart disease. JAMA 1999;282:2169ŌĆō2171.


117. Siti HN, Kamisah Y, Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascul Pharmacol 2015;71:40ŌĆō56.


118. Danesh J, Whincup P, Walker M, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ 2000;321:199ŌĆō204.



119. Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001;286:327ŌĆō334.


120. Duncan BB, Schmidt MI, Pankow JS, et al. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes 2003;52:1799ŌĆō1805.

121. Fuentes E, Fuentes F, Vilahur G, Badimon L, Palomo I. Mechanisms of chronic state of inflammation as mediators that link obese adipose tissue and metabolic syndrome. Mediators Inflamm 2013;2013:136584.



122. Catrysse L, van Loo G. Inflammation and the metabolic syndrome: the tissue-specific functions of NF-╬║B. Trends Cell Biol 2017;27:417ŌĆō429.


123. Tajti G, Gesztelyi R, Pak K, et al. Positive correlation of airway resistance and serum asymmetric dimethylarginine level in COPD patients with systemic markers of low-grade inflammation. Int J Chron Obstruct Pulmon Dis 2017;12:873ŌĆō884.



124. Robinson WH, Lepus CM, Wang Q, et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol 2016;12:580ŌĆō592.



125. von K├żnel R, Hepp U, Kraemer B, et al. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res 2007;41:744ŌĆō752.


126. Speer K, Upton D, Semple S, McKune A. Systemic low-grade inflammation in post-traumatic stress disorder: a systematic review. J Inflamm Res 2018;11:111ŌĆō121.



127. Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry 2010;67:446ŌĆō457.


128. Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience 2013;246:199ŌĆō229.



129. R├Čnnb├żck C, Hansson E. The importance and control of low-grade inflammation due to damage of cellular barrier systems that may lead to systemic inflammation. Front Neurol 2019;10:533.


130. Miwa H, Oshima T, Tomita T, et al. Recent understanding of the pathophysiology of functional dyspepsia: role of the duodenum as the pathogenic center. J Gastroenterol 2019;54:305ŌĆō311.

131. Lubbers ER, Price MV, Mohler PJ. Arrhythmogenic substrates for atrial fibrillation in obesity. Front Physiol 2018;9:1482.



132. Van't Klooster CC, Ridker PM, Hjortnaes J, et al. The relation between systemic inflammation and incident cancer in patients with stable cardiovascular disease: a cohort study. Eur Heart J 2019;40:3901ŌĆō3909.



133. Ahechu P, Zozaya G, Mart├Ł P, et al. NLRP3 inflammasome: a possible link between obesity-associated low-grade chronic inflammation and colorectal cancer development. Front Immunol 2018;9:2918.



134. Schuckit MA. Alcohol, anxiety, and depressive disorders. Alcohol Health Res World 1996;20:81ŌĆō85.


135. Lu Y, Jin X, Feng LW, Tang C, Neo M, Ho RC. Effects of illness perception on negative emotions and fatigue in chronic rheumatic diseases: rumination as a possible mediator. World J Clin Cases 2022;10:12515ŌĆō12531.



136. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep 2021;70:1ŌĆō187.



137. Derbyshire KL, Grant JE. Compulsive sexual behavior: a review of the literature. J Behav Addict 2015;4:37ŌĆō43.



138. Marks LR, Nolan NS, Liang SY, Durkin MJ, Weimer MB. Infectious complications of injection drug use. Med Clin North Am 2022;106:187ŌĆō200.


139. Mirin SM, Meyer RE, McNamee HB. Psychopathology and mood during heroin use: acute vs chronic effects. Arch Gen Psychiatry 1976;33:1503ŌĆō1508.


140. Tomaka J, Blascovich J, Kibler J, Ernst JM. Cognitive and physiological antecedents of threat and challenge appraisal. J Pers Soc Psychol 1997;73:63ŌĆō72.


141. Blackburn EH, Epel ES, Lin J. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science 2015;350:1193ŌĆō1198.


142. Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr 2006;84:475ŌĆō482.


143. Lacey J, Corbett J, Forni L, et al. A multidisciplinary consensus on dehydration: definitions, diagnostic methods and clinical implications. Ann Med 2019;51:232ŌĆō251.



144. Johnson KVA. Gut microbiome composition and diversity are related to human personality traits. Hum Microb J 2020;15:100069.



145. Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci 2008;1141:105ŌĆō130.



146. Anand P, Kunnumakkara AB, Sundaram C, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res 2008;25:2097ŌĆō2116.



147. Blackburn E, Epel E. The telomere effect: a revolutionary approach to living younger, healthier, longer. New York: Grand Central Publishing, 2017.
Inflammatory pathway components. The inflammatory pathway consists of inducers, sensors, mediators, and target tissues. Inducers initiate the inflammatory response and are detected by sensors. Sensors, such as TLRs, are expressed on specialized sentinel cells, such as tissue-resident macrophages, dendritic cells, and mast cells. They induce the production of mediators, including cytokines, chemokines, bioactive amines, eicosanoids, and products of proteolytic cascades, such as bradykinin. These inflammatory mediators act on various target tissues to elicit changes in their functional states that optimize adaptation to the noxious condition (e.g., infection or tissue injury) associated with the particular inducers that elicited the inflammatory response. The specific components shown represent only a small sample of the myriad different sensors, mediators, and target tissues involved in the inflammatory response. Reproduced from Medzhitov [2]. TLR, Toll-like receptor; TNF, tumor necrosis factor; IL, interleukin; CCL2, C-C motif chemokine ligand 2; CXCL8, C-X-C motif chemokine ligand 8.
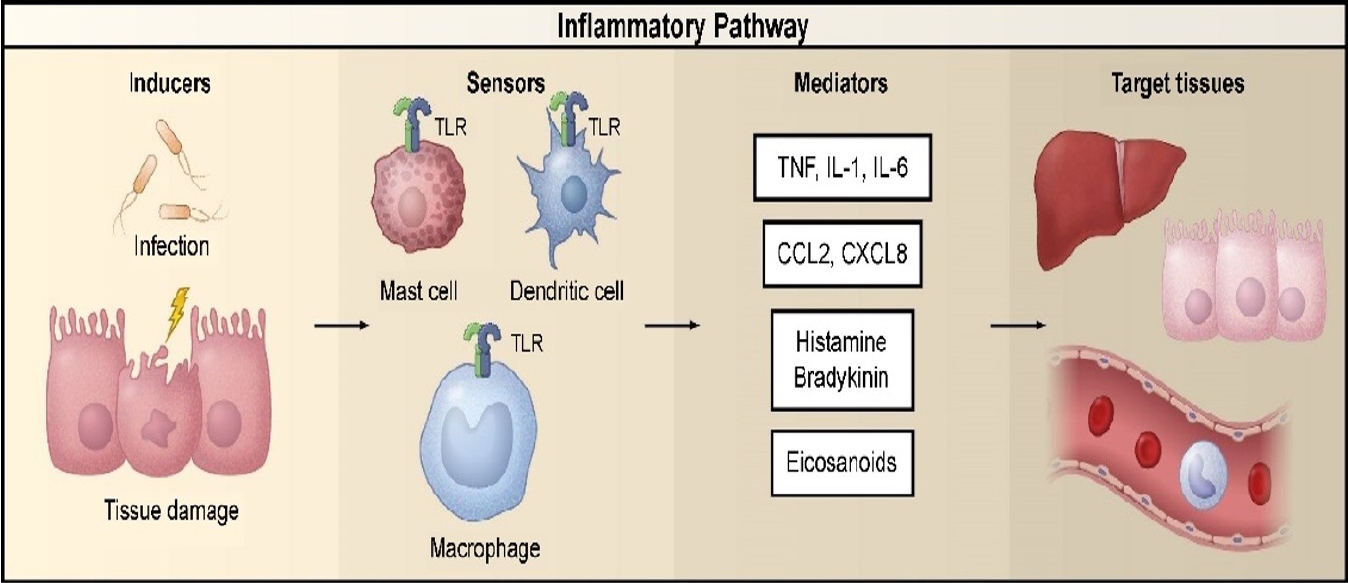
Figure┬Ā1.
A model of disease development that integrates human understanding. Stress hormones mean glucocorticoids-epinephrinenorepinephrine. Exo-SI, exogenous substance of inducer; HPA, hypothalamic-pituitary-adrenal; SNS, sympathetic nervous system; IM, inflammatory mediator; Endo-SI, endogenous substance of inducer.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print