 |
 |
| Korean J Med > Volume 97(4); 2022 > Article |
|
Abstract
Chimeric antigen receptor (CAR) T-cell therapy constitutes a revolutionary advancement in personalized cancer treatment. During this treatment, a patient's own T cells are genetically engineered to express a synthetic receptor that binds a tumor antigen. CAR-T cells are then expanded for clinical use and infused back into the patient's body to attack cancer. CAR-T cells have produced remarkable clinical responses with B-cell malignancies. However, CAR-T cells therapy is not without problems. Barriers to effective CAR-T cells therapy include severe life-threatening toxicities and modest anti-tumor activity. In this review, we introduce the concept of CAR-T cells therapy, currently available CAR-T cells therapy options, and how to deal with adverse events.
수십 년 동안 암 치료의 기초는 수술, 화학 요법 및 방사선 요법이었다. 하지만 지난 10년간 새로운 개념의 치료법이 암 치료의 그림을 바꾸고 있으며, 그중 면역 치료(immunotherapy; 암을 공격하는 환자의 면역 체계의 힘을 동원하고 강화하는 요법)가 여러 가지 암종에서 표준 치료법으로 자리 잡았다[1]. CAR-T 세포 치료제 또한 면역 치료 중 하나로 2017년부터 6개의 치료제가 미국 Food and Drug Administration (USFDA) 승인을 받아 사용 중이다(Table 1) [2-8].
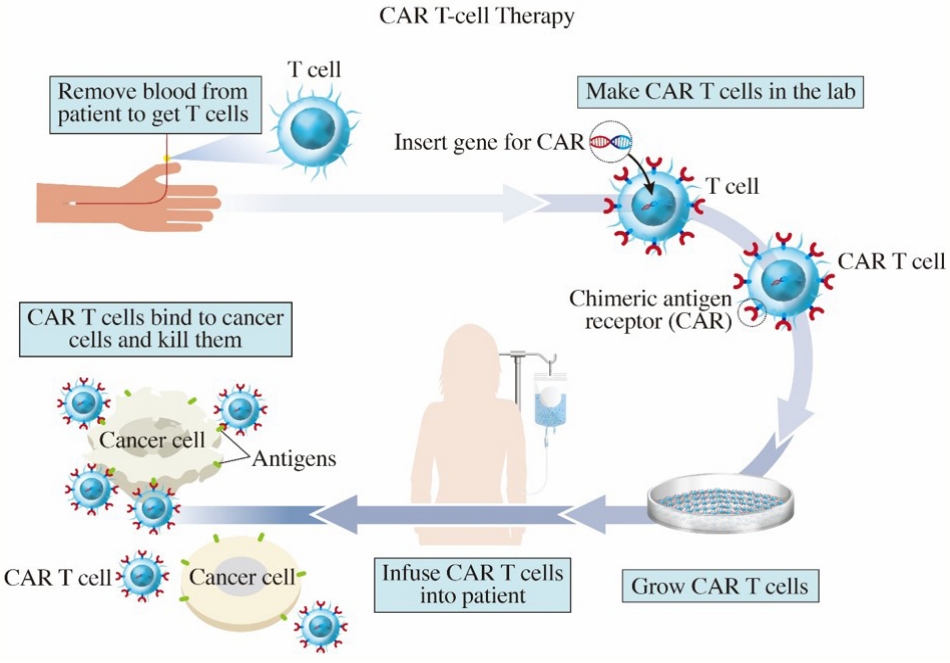
CAR-T는 환자의 혈액에서 T세포를 뽑아 암세포를 잘 인식할 수 있도록 유전자 조작을 거친 뒤 배양하여 다시 환자의 몸에 투약하는 개인 맞춤형 의약품이다(Fig. 1). 일반적으로 세포 면역계는 수지상세포를 통해 가공된 항원을 T세포가 T세포 수용체(T cell receptor, TCR)를 통해 인식하여 활성화되는 복잡한 방식으로 작동한다. 이와는 달리 CAR-T는 표면 항원을 잘 인식한다고 알려진 항체를 사용하는 방법을 쓰게 되는데, T세포는 종양을 인식하는 데 항체를 사용하지 않기 때문에 강제로 항체 유전자를 T세포에 넣고 T세포가 활성화되는 후속 과정에 필요한 세포 내 분자들도 항체와 연결해 TCR을 대체하는 ‘결합된(chimeric) 항원(antigen) 수용체(receptor)’, 즉, CAR이라는 방식을 쓰게 된다. CAR은 4가지 주요 구성요소로 이루어져 있는데, 1) 세포외 표적 항원 결합 도메인(extracellular target antigen-binding domain), 2) 힌지 영역(hinge region), 3) 막횡단 도메인(transmembrane domain) 및 4) 하나 이상의 세포 내 신호전달 도메인(one or more intracellular signaling domains)이 그것이다. 1세대 CAR의 경우 세포내 신호전달 도메인에 co-stimulatory molecule이 없어 단순히 일시적인 T세포 증식 및 제한된 사이토카인 분비를 유도하여 그 효과가 미미하였다. 현재 널리 사용 중인 2세대 CAR의 경우, CD28 또는 4-1BB와 같은 co-stimulatory molecule이 세포내 신호전달 도메인에 통합되어 생체 내에서 CAR-T 세포의 생존과 기능을 촉진하여 효과가 극대화되었다[9-11]. CAR-T 세포 치료제 특성상 암세포 표면에 명확한 표적 항원이 있어야 해서, 현재 승인 받은 치료제들은 B세포의 CD19 [12]와 B세포 성숙 항원(B-cell maturation antigen, BCMA) [13]을 표적으로 한다.
앞에서 언급하였듯이 CAR-T 세포를 유전공학적으로 조작하기 위해 먼저 암환자에서 T세포를 채집한다(Fig. 1). 이 T세포에 바이러스를 이용한 형질도입 기법을 통해 암세포에만 반응하는 특이적인 수용체를 환자의 T세포에 발현시키도록 하는 새로운 유전 정보를 주입하고, 이 T세포들을 배양하여 대량으로 증식시킨다. 이 과정은 대략 1달 정도 소요되는데, 이 기간 동안 환자는 필요에 따라 가교 치료(bridging therapy)를 받기도 한다. CAR-T 세포 치료제가 완성이 되면 전처치 요법 후 세포 주입을 하게 된다. 이로써 정상 세포 손상은 최소화 하면서 CAR을 가진 T세포가 효과적으로 암 세포를 사멸하도록 한다. T세포에 만들어진 단백질인 CAR은 세포 외부에서 암세포를 인식하는 역할을 하고 세포 내부에서는 T세포를 활성화하는 신호를 전달하여 암세포를 공격하는 역할을 한다.
현재 USFDA 승인을 받은 CAR-T 세포 치료제를 사용할 수 있는 림프종의 종류는 광범위큰B세포림프종(diffuse large B cell lymphoma, DLBCL)과 외투세포림프종(mantle cell lymphoma, MCL)이 있다. 이 중에서도 특히 DLBCL의 경우, 최근 치료에 있어 가장 빠르고 다양한 변화가 있었던 혈액암으로, 2021년을 기점으로 우리나라에서도 tisagenlecleucel 치료가 가능해졌다.
Axicabtagene ciloleucel으로 재발불응성 DLBCL에서 시행한 ZUMA-1 [2,14] 임상시험에서 완전관해율 및 12개월 무진행 생존율은 각각 58% 및 44%였으며, tisagenlecleucel로 치료한 DLBCL에서 중추적인 JULIET [5] 임상시험의 완전관해율은 약 40%였다. 이러한 결과는 표 2에 요약되어 있다.
Tisagenlecleucel의 경우 현존하는 가장 효과적인 재발불응성 DLBCL의 치료법이다. 다만, 2차요법으로 사용하기 위한 3상 임상시험에서 우월하지 못한 결과를 보여주어[15], axicabtagene ciloleucel, lisocabtagene maraleucel 등 경쟁 CAR-T 제품과의 비교우위를 점할 수 있을지에 대한 우려를 자아내고 있다. Axicabtagene ciloleucel과 lisocabtagene maraleucel의 경우 2차요법으로 사용하기 위한 임상시험에서 tisagenlecleucel와 다르게[16] 기존 치료법 대비 우월한 결과를 보여주어[14,16,17], tisagenlecleucel에 비하여 후발주자임에도 불구하고 재발불응성 DLBCL의 새로운 치료제로서의 성공적인 발판을 마련하였다.
다발골수종에 대한 CAR-T 세포 치료제 연구는 림프종 및 ALL에 비하면 아직 초기 단계이다. 앞서서도 언급되었듯이 가장 널리 연구된 골수종 CAR 표적은 BCMA로 이는 종양 괴사 인자 수용체 슈퍼패밀리 구성원 17 (tumor necrosis factor receptor super family member 17, TNFRSF17)이다. BCMA는 형질 세포에서는 우선적으로 발현되지만 조혈 줄기 세포에서는 발현되지 않기 때문에 이상적인 표적 항원이라 할 수 있으며, 리간드인 B-세포 활성화 인자(B-cell activating factor, BAFF) 및 증식 유도 리간드(a proliferation-inducing ligand, APRIL)의 BCMA에 대한 결합 및 자극은 골수에서 형질 세포의 성장 및 증식을 촉진한다[19-21].
BCMA CAR-T 세포 치료제의 선두주자는 idecabtagene vicleucel [7]로, SFDA에서 breakthrough-therapy designation award를 받으며 다발골수종에서도 CAR의 시대를 열었다. 그 후 항원 결합 친화도를 향상시키기 위해 2개의 BCMA 표적화 도메인을 포함하는 이중 epitope CAR-T 세포 제품인 JNJ-4528도 CARTITUDE-1 [22] 임상시험에서 인상적인 94.8%의 overall response rate를 보여주었다. 이 연구는 CAR-T 제품의 단일 저용량 주입이 조기에 깊고 지속적인 반응을 유도하기에 충분하다는 강력한 증거를 제공하였다. 다른 주목할만한 BCMA CAR-T 제품은 이식편대숙주병을 줄이고 T세포의 지속성을 연장하도록 설계된 인간 유래 및 완전 인간화 CAR (JCARH125) [22,23]이다. JCARH125의 효능은 CD3+ 순도가 높고 초기 기억 표현형 및 다관용성이 풍부한 T세포 제품과 관련이 있는 것으로 보고되었다(Table 3).
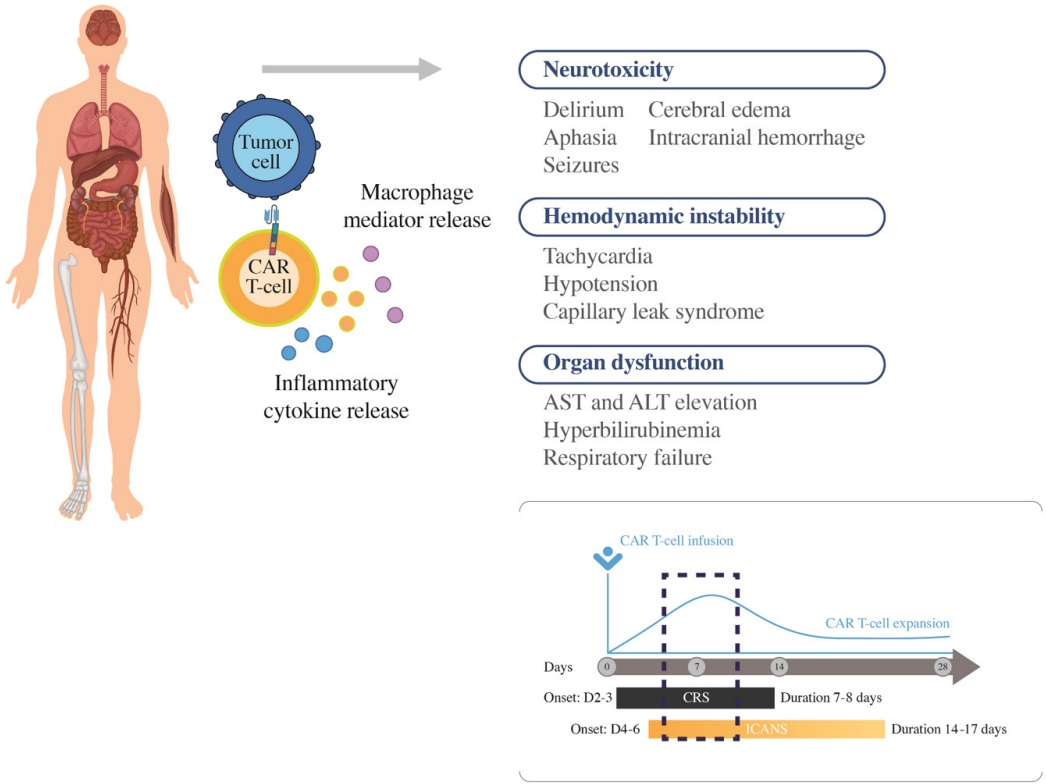
가장 주목해야 하는 부작용으로는 사이토카인 분비 증후군(cytokine release syndrome, CRS)과 신경독성 증후군(immune effector cell associated neurotoxicity syndrome, ICANS)이 있다[24]. 그 빈도는 각 CAR-T 세포 치료제의 용량, CAR의 구조(e.g CD28 costimulatory molecule 사용 시 높은 빈도로 발생), 병의 종류 및 정도, 환자의 상태에 따라 달라질 수 있다(Fig. 3).
CRS의 경우 CAR-T의 활성화 및 증식과 함께 염증성 사이토카인(IL-1β, IL-6, IL-10, chemokines [CXCL8, CCL2], interferon-γ, granulocyte-macrophage colony-stimulating factor and tumor necrosis factor)의 분비 증가로 인해 생기는 것으로, 대부분 CAR-T 세포 치료제 주입 후 2-3일 내 발생하여 7-8일 정도 지속되는 것으로 알려져 있다[25]. 치료를 요하는 CRS의 빈도는 7-47% 정도로 보고되어 있다. CRS의 병태생리는 다섯 가지 주요 단계로 나눌 수 있다. CRS와 관련된 증상 및 징후에는 고열, 오한, 경직, 동성 빈맥, 근육통 및 피로가 대표적이다. 저혈압, 저산소증, 부정맥, 심장 박출률 저하 및 기타 말단 기관 기능 장애와 같은 더 심각한 독성도 발생할 수 있다. 또한 CRS와 관련된 장기 독성에는 가역적 신부전, 간 효소 상승, 전해질 고갈, 혈구감소증 및 응고병증 등이 있다. CRS Grading system은 여러 가지가 있으나 가장 널리 사용되는 것은 Lee 등[24]이 보고한 American Society for Transplantation and Cellular Therapy (ASTCT) grading으로 표 4에 정리되어 있다.
반면 ICANS를 포함한 신경독성의 경우, 혈액뇌장벽을 통과한 T세포, 단핵구 등에 의하여 분비된 사이토카인이 관여하는 것으로 알려져 있으며, CRS보다 조금 늦은 시기인 CAR-T 세포 치료제 주입 후 4-6일에 발생하여 14-17일 정도 지속되는 것으로 알려져 있다. ICANS의 초기 증상에는 표현성 실어증, 떨림, 난시 및 혼수가 있다. 이러한 증상은 전체 실어증, 발작, 둔감, 혼미 및 혼수 상태로 진행될 수 있다.
표 5에 CRS와 ICANS의 치료가 정리되어 있다. 낮은 등급의 CRS는 감염과 같은 발열의 다른 동반 원인이 없는지 확인하면서 해열제를 통한 지지요법으로 관리한다. 중등도에서 중증 CRS는 IL-6R 차단 항체인 토실리주맙(tocilizumab)과 함께 필요 시 코르티코스테로이드를 함께 사용한다. 낮은 등급의 ICANS는 또한 일반적으로 진단 정밀 검사와 지지요법으로 관리되는 반면, 중증 ICANS는 대개 대부분의 경우, 코르티코스테로이드 치료를 하게 된다. 토실리주맙의 사용은 심각한 CRS의 발병률을 현저히 감소시켰는데, IL-6가 downstream inflammatory cascade의 핵심 매개체이며, CRS 초기에 최고조에 다다르기 때문이다. 다만 코르티코스테로이드는 CAR-T 세포 및 기타 방관자 면역 세포, 특히 골수 세포의 증식 및 사이토카인 분비 억제를 포함하여 전체적인 면역 억제를 유도할 수 있기 때문에 심각한 상황에서만 함께 고려를 해야 한다.
토실리주맙의 경우, CRS의 관리에 매우 효과적이지만 ICANS의 경우 대부분에서효과가 없다. 이는 CRS와 ICANS 사이의 병태생리학적 차이 및/또는 BBB를 통한 토실리주맙의 빈약한 침투 때문이라고 생각한다. 실제로, 토실리주맙의 예방적 사용은 심각한 CRS의 발생을 감소시켰지만 되려 심각한 ICANS의 발생을 증가시켰는데, 이는 아마도 토실리주맙 투여 후 관찰되는 혈청 IL-6 수치의 감소로 인해 수용체 차단에 의해 말초 조직으로의 흡수를 방지하였기 때문이다. 이에 ICANS 치료에 근간이 되는 것은 코르티코스테로이드이다. 대부분의 연구에 따르면 토실리주맙의 사용은 CAR-T 세포의 효능에 영향을 미치지 않는 것으로 보이나, 코르티코스테로이드의 사용은 조기 진행 및 사망 위험이 증가하는 불리한 임상 결과를 보인 바 있기 때문에 CRS 및 ICANS의 병태생리학적 메커니즘에 대한 더 나은 이해와 이들의 관리를 위한 새로운 전략 개발이 필요하다[25].
CAR-T 세포 치료의 장기 독성으로는 감염 위험을 증가시킬 수 있는 저감마글로불린혈증 및 혈구감소증을 유발하는 B세포 무형성이 포함된다. 악성 B세포와 정상 B세포 모두 표면에서 CD19를 발현하여 항-CD19 CAR-T 세포 요법 후 "on-target, off-tumor" B세포 무형성을 유발할 수 있다. B세포 무형성은 잠재적으로 수개월에서 수년 동안 지속될 수 있으며 빈번한 감염으로 이어질 수 있기 때문에, 필요 시 면역글로불린 치료가 필요하다. 더불어 면역 매개 범혈구감소증을 유발할 수 있기 때문에 최소 6개월 이상 추적 관찰을 하며 적정한 수혈 및 성장 인자 지원이 필요하다[26-28].
REFERENCES
1. June CH, O'Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science 2018;359:1361–1365.


2. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;377:2531–2544.



3. Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2019;380:45–56.


4. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018;378:439–448.



5. Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 2020;396:839–852.


6. Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2020;382:1331–1342.



7. Munshi NC, Anderson LD Jr, Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med 2021;384:705–716.


8. Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med 2019;380:1726–1737.



9. Brentjens RJ, Latouche JB, Santos E, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med 2003;9:279–286.


10. Mohanty R, Chowdhury CR, Arega S, Sen P, Ganguly P, Ganguly N. CAR T cell therapy: a new era for cancer treatment (review). Oncol Rep 2019;42:2183–2195.


11. Fischer JW, Bhattarai N. CAR-T cell therapy: mechanism, management, and mitigation of inflammatory toxicities. Front Immunol 2021 Jun 18 [Epub]. https://doi.org/10.3389/fimmu.2021.693016


12. Wang K, Wei G, Liu D. CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Exp Hematol Oncol 2012;1:36.



13. Cho SF, Anderson KC, Tai YT. Targeting B cell maturation antigen (BCMA) in multiple myeloma: potential uses of BCMA-based immunotherapy. Front Immunol 2018;9:1821.

14. Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol 2019;20:31–42.

15. ClinicalTrials.gov.Tisagenlecleucel in adult patients with aggressive B-cell non-Hodgkin lymphoma (BELINDA) [Internet]. Bethesda (MD): ClinicalTrials.gov, c2021 [cited 2021 Aug 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT03570892
16. Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US lymphoma CAR T consortium. J Clin Oncol 2020;38:3119–3128.



17. Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med 2022;386:640–654.


18. ClinicalTrials.govA study to compare the efficacy and safety of JCAR017 to standard of care in adult subjects with high-risk, transplant-eligible relapsed or refractory aggressive B-cell non-Hodgkin lymphomas (TRANSFORM) [Internet]. [Internet]. Bethesda (MD): ClinicalTrials.gov, c2022 [cited 2021 Aug 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT03575351
19. Moreaux J, Legouffe E, Jourdan E, et al. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood 2004;103:3148–3157.



20. Sanchez E, Li M, Kitto A, et al. Serum B-cell maturation antigen is elevated in multiple myeloma and correlates with disease status and survival. Br J Haematol 2012;158:727–738.


21. Mikkilineni L, Kochenderfer JN. CAR T cell therapies for patients with multiple myeloma. Nat Rev Clin Oncol 2021;18:71–84.


22. Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet 2021;398:314–324.


23. ClinicalTrials.govStudy evaluating the safety and efficacy of JCARH125 in subjects with relapsed and/or refractory multiple myeloma (EVOLVE) [Internet]. Bethesda (MD): ClinicalTrials.gov, c2022 [cited 2021 Aug 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT03430011
24. Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 2019;25:625–638.


25. Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014;124:188–195.



26. Du M, Hari P, Hu Y, Mei H. Biomarkers in individualized management of chimeric antigen receptor T cell therapy. Biomark Res 2020;8:13.



27. Adkins S. CAR T-cell therapy: adverse events and management. J Adv Pract Oncol 2019;10(Suppl 3):21–28.
Table 1.
Table 2.
CAR-T cell therapies for refractory DLBCL treatment
| ZUMA-1 [2] | JULIET [5] | TRANSCEND NHL 001 [6] | |
|---|---|---|---|
| Name | Axicabtagene ciloleucel | Tisagenlecleucel | Lisocabtagene maraleucel |
| Kite Pharma YESCARTA® | Novartis KYMRIAH® | Juno Therapeutics BREYANZI® | |
| Structure | Anti-CD19-CD28-CD3z | Anti-CD19-41BB-CD3z | Anti-CD19-41BB-CD3z |
| Patients pheresed/treated | 111/101 | 165/111 | 344/269 |
| Bridging therapy | None allowed in pivotal trial | 92 | 59 |
| ORR (CR) | 82 (54.0) | 52 (40.0) | 73 (53.0) |
| Cell dose | 2×106/kg (max 2×108) | 0.6 to 6.0×108/kg | 50 to 150×106/kg |
| Lymphodepletion | Flu/Cy 30/500 mg/m2×3 days | Flu/Cy 25/250 mg/m2×3 days, or bendamustine×2 days | Flu/Cy 30/300 mg/m2×3 days |
Table 3.
Efficacy and safety of BCMA CAR-T-cell therapies
| KaRMMa [7] | EVOLVE [22] | CARTITUDE-1 [21] | |
|---|---|---|---|
| Name | Idecabtagene vicleucel | Orvacabtagene autoleucel | Ciltacabtagene autoleucel |
| Celgene ABECMA® | JCARH125 | Jassen CARVYKTI® | |
| Structure | Anti-BCMA, 4-1BB, CD3z | Anti-BCMA, 4-1BB, CD3z | Anti-BCMA, 4-1BB, CD3z (2 epitopes) |
| Vector | Lentivirus | Fully humanized | Lentivirus |
| Number of patients enrolled | 128 | 62 | 97 |
| ORR (CR) | 73 (33.0) | 92 (36.0) | 100 (67.0) |
| PFS (months) | 8.8 | NR | NR |
| DoR (months) | 10.7 | NR | NA |
| CRS (≥ Grade 3) | 84 (6.0) | 89 (3.0) | 95 (5.0) |
| Median time to CRS (days) | 1 (1-12) | 2 (1-4) | 7 (1-12) |
| Median duration of CRS (days) | 5 (1-63) | 4 (1-10) | 4 (1-97) |
| ICANS (≥ Grade 3) | 17 (3.0) | 13 (3.0) | 17 (2.0) |
Values are presented as number (%) or median (interquartile range).
BCMA, B-cell maturation antigen; CAR, chimeric antigen receptor; ORR, overall response rate; CR, complete remission; PFS, progression progression-free survival; DoR, duration of response; CRS, cytokine release syndrome; ICANS, immune effector cell cell-associated neurotoxicity syndrome.
Table 4.
Efficacy and safety of BCMA CAR-T cell therapies
Table 5.
Summary of CRS and neurotoxicity management
| Grade | CRS | Neurotoxicity | CRS + neurotoxicity |
|---|---|---|---|
| 1 | Supportive care (consider tocilizumab in select casesa) | Supportive care (consider tocilizumab in select casesa) | Supportive care |
| 2 | Tocilizumab | Steroids (dexamethasone or methylprednisolone) | Tocilizumab + steroids (dexamethasone) |
| 3 | Tocilizumab | Steroids (dexamethasone) | Tocilizumab + steroids (dexamethasone) |
| 4 | Tocilizumab + high dose steroids (methylprednisolone) | High-dose steroids (methylprednisolone) | Tocilizumab + high dose steroids (methylprednisolone) |
| ICU/critical care | ICU/critical care | ICU/critical care |
-
METRICS

-
- 1 Crossref
- 0 Scopus
- 31,343 View
- 1,029 Download




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



