 |
 |
| Korean J Med > Volume 96(5); 2021 > Article |
|
요약
목적
단일 상급종합병원에 보고된 1차 항결핵제의 약물이상반응 정보를 통해 한국인에서 항결핵제 약물이상반응 발생 현황을 파악하여 향후 결핵 관리를 위한 약물이상반응 대책 수립에 활용한다.
방법
2009년부터 2018년까지 3차 의료기관에서 1차 항결핵제인 이소니아지드, 리팜핀, 에탐부톨, 피라진아미드를 처방 받은 환자들에 대해 원내 보고된 약물이상사례 데이터베이스를 기반으로 약물이상반응 정보를 수집하였고 약물이상반응이 발생한 환자의 나이, 성별, 총 치료 기간과 약물이상반응의 발생시점, 장기별 분류, 중증도 및 심각도 등을 분석하였다.
결과
항결핵제를 처방 받은 5,482명 중 1,606명(29.3%)에서 약물이상반응이 보고되었고, 한 환자에서 1건에서 많게는 5건까지 총 2,098건의 약물이상반응이 보고되었는데, 경증이 680건(32.4%), 중등증이 1,282건(61.1%), 중증이 136건(6.5%)이었고 심각한 약물이상반응은 127건(6.1%)이었다. 1차 항결핵제에 의한 약물이상반응의 발생 장기별 분포는 피부 및 부속기관 장애가 27.5%로 가장 많았고, 위장관계 장애(17.5%)와 간 및 담도계 질환(13.1%)이 그 뒤를 이었다. 처방 건 당 이상반응 보고는 피라진아미드가 29.6% (1,406건/4,757명)로 가장 높았다. 중증 약물이상반응은 간 및 담도계 질환이, 심각한 약물이상반응의 경우 전신적 질환이 가장 많이 보고되었다. 총 치료 기간을 비교한 결과 약물이상반응이 발생하지 않았던 군은 224.0 ± 3.1일, 약물이상반응이 발생하였던 군은 247.0 ± 4.7일로 약물이상반응이 발생한 군이 통계적으로 유의하게 치료 기간이 평균 23일 정도 길었다(p = 0.009).
Abstract
Background/Aims
Tuberculosis has incidence and mortality rates that are among the highest for all communicable diseases. Adverse drug reactions (ADRs) to anti-tubercular drugs are common, and have a major impact on treatment maintenance and prognosis. It is important to understand the characteristics of ADRs and establish a suitable management plan.
Methods
We retrospectively reviewed patients with ADRs during treatment with first-line antitubercular drugs such as isoniazid, rifampicin, ethambutol, and pyrazinamide from 2009 to 2018. Age, sex, and total treatment period, and the onset, severity, seriousness, and system organ class of ADRs, were analyzed to understand the characteristics of first-line anti-tubercular drug-related ADRs.
Results
A total of 1,606 of 5,482 patients (29.3%) experienced ADRs after administration of first-line anti-tubercular drugs. The incidence of ADRs related to isoniazid, rifampicin, ethambutol, and pyrazinamide was 22.2%, 21.3%, 24.5%, and 29.6%, respectively. A total of 2,098 ADR reports were made (mean of 1.3 ± 0.6 per patient). The rates of mild, moderate, and severe ADRs were 32.4%, 61.1%, and 6.5%, respectively. There were 127 reports (6.1%) of serious ADRs. Skin and appendage disorders were most frequently reported (27.5%), followed by gastrointestinal disorders (17.5%), and liver and biliary system disorders (13.1%). The total treatment period was longer in patients who experienced ADRs (224.0 ± 3.1 days vs. 247.0 ± 4.7 days, p = 0.009).
결핵은 단일 감염원에 의한 사망의 10대 원인 중 하나로, 2018년 전 세계적으로 약 1,000만 명의 환자가 발생하였고 150만 명이 결핵으로 사망하였다[1]. 우리나라의 결핵 발생률은 2012년부터 지속적으로 감소하고 있으나, 여전히 OECD 가입국 중 결핵 발생률 및 유병률 1위, 사망률 2위를 차지하였다[2].
결핵의 초치료는 일반적으로 2개월의 초기 집중 치료기와 4개월의 유지 치료기로 이루어진다. 일반적으로 초기 집중 치료기에는 이소니아지드, 리팜핀, 에탐부톨, 피라진아미드의 4제를 투약하고, 유지 치료기에는 이소니아지드, 리팜핀, 에탐부톨의 3제 투약이 권고된다[3]. 결핵 치료는 동시에 여러 가지 약제를 장기간 사용하고, 이로 인한 부작용 위험이 높아 복약순응도가 떨어질 우려가 있다. 복약순응도가 낮을 경우 결핵균이 약제에 대한 내성을 획득하여 치료 효과를 떨어뜨리게 된다. 결핵 치료에 있어 복약순응도가 중요한 만큼, 환자가 꾸준한 약제 복용에 대한 필요성을 인지하도록 교육해야 하며, 약제 복용 후 발생할 수 있는 약물이상반응 및 대처방안 정보를 복약지도를 통해 사전에 제공하는 것이 필요하다.
1차 항결핵제는 사용된 지 30년 이상이 되었음에도 불구하고[4], 이들 약제에 의한 약물이상반응의 구체적인 역학 정보는 전 세계적으로도 많지 않다. 다양한 문헌에서 보고된 약물이상반응은 각각 연구 기간이나 대상 선정 기준이 상이하여, 1차 항결핵제 약물이상반응 빈도를 정확하게 대변하기 어렵다. 대상수집의 경우 짧게는 1년[4,5]에서부터 길게는 14년[6]까지 이루어졌으며, 연구 대상도 활동성 결핵 환자이거나[7] 호흡기 결핵 환자이거나[8] 광범위 약제내성 결핵 환자를 대상으로 하는 등[9] 다양하였다. 또한 유전자 변이에 따라 약제의 독성에 차이가 나타나며[10], 항결핵제에 의한 간독성이 성별, 인종, acetylator 발현 정도 등에 의한 차이가 나타날 수 있다고 알려져 있다[11]. 이에 한국인에서의 항결핵제 관련 정확한 이상반응 자료를 확보한다면 국내 결핵의 성공적인 관리를 위한 참고자료로 활용할 수 있을 것이다.
본 연구는 2009년 1월부터 2018년 12월까지 서울대학교병원에서 국제질병분류(ICD-10) 상 결핵(A15-A19)으로 진단받고 1차 항결핵제(이소니아지드, 리팜핀, 에탐부톨, 피라진아미드)를 처방 받은 환자를 대상으로 하였으며, 서울대학교병원 윤리심의위원회의 승인을 받고 진행되었다(IRB No.1412-044-632).
서울대학교병원 임상 연구 분석시스템인 SUPREME을 활용하여, 본 연구의 대상 환자 수 및 이들이 사용한 1차 약제의 종류 및 사용 기간에 대한 정보를 수집하였다. 결핵을 진단받고 항결핵제 처방을 받은 환자들은 외래 추적 관찰 때마다 결핵 관리 전담간호사와 상담을 통해 복약 여부 및 부작용 발생 여부를 확인하며, 이때 확인한 부작용들은 서울대학교병원 약물안전센터에 신고되어 개별 약물 이상사례 보고자료(individual case safety report, ICSR)가 작성된다. ICSR은 약물이상반응의 발생 시기, 원인약제, 중증도, 심각도, 인과성 정보를 포함하며, 약물이상반응의 중증도, 심각도, 인과성은 약물안전센터의 전담약사 및 전담간호사에 의해 다음의 기준과 같이 평가되었다.
약물이상반응의 중증도는 경증(mild), 중등증(moderate), 중증(severe) 3단계로 구분하였으며, 경증은 증상 또는 증후를 지각할 수는 있으나 약제를 그대로 유지할 수 있는 정도, 중등증은 약제가 변경, 중단되거나 증상에 대한 치료가 필요한 정도, 중증은 일이나 일상의 활동을 수행할 수 없는 정도로 정의하였다[12]. 심각도는 심각함(serious) 또는 심각하지 않음(not serious)으로 구분하였고, 사망, 즉각적으로 생명을 위협하는 경우, 영구적 또는 실질적 불구나 기능저하를 초래하는 경우, 선천적 기형을 유발한 경우 등을 심각함으로 정의하였다[13]. 인과성의 경우 세계보건기구 웁살라 모니터링센터(World Health Organization-Uppsala Monitoring Centre, WHO-UMC)의 인과성 평가 기준으로 확실(certain), 가능성 높음(probable), 가능성 있음(possible)으로 평가하였으며[14], 인과성 낮음(unlikely)으로 평가된 경우는 분석에서 제외하였다.
평가된 ICSR의 원인약제와 인과성, 발생한 약물이상반응의 증상 및 증상의 중증도와 심각도를 분석하여 1차 항결핵제에 의한 약물이상반응의 특징을 파악하였다.
대상 환자 중 약물이상반응이 발생한 환자군과 그렇지 않은 환자군에서 1차 항결핵제를 사용한 총 기간을 비교하여, 약물이상반응 발생 여부와 결핵의 치료 기간에 연관성이 있는지 확인하였다.
본 기관에서 10년 간 결핵으로 진단되어 1차 항결핵제를 처방 받은 환자는 총 5,482명이었고, 남자가 2,902명으로 52.9%였다. 연령대로 보았을 때에는 60대에서 1,019건(18.6%), 50대에서 1,013건(18.5%) 순으로 많았다(Fig. 1). 진단명으로 보았을 때 호흡기 결핵(A15, A16)으로 진단받은 환자가 3,856명으로 70.3%를 차지하였다.
약물이상반응은 1,606명(29.3%)에서 총 2,098건의 ICSR이 보고되었는데, 발생률은 남녀간 차이가 없었으며, 연령대별로는 50대에서 31.8%로 가장 높았다. ICSR은 한 환자 당 1건에서 많게는 5건(1.3 ± 0.6)까지 보고되었다. WHO-UMC 인과성 평가에 의한 약물이상반응의 인과성은 가능성 있음이 1,208건(57.6%)으로 가장 많았고, 가능성 높음이 862건(41.1%), 그리고 확실함이 28건(1.3%)이었다.
약물이상반응의 중증도는 중등증이 1,282건(61.1%)으로 가장 많았고, 경증 680건(32.4%), 중증 136건(6.5%)이었다. 심각도를 기준으로 하였을 때 심각한 사례는 127건(6.1%)이었다.
약물이상반응의 원인약제로 의심되어 신고된 각 약제별 사례는 피라진아미드가 1,406건으로 가장 많았고, 에탐부톨 1,259건, 이소니아지드 1,173건, 리팜핀 1,116건이었다. 약제사용량을 고려한 약물이상반응의 발생률은 피라진아미드가 29.6% (1,406건/4,757명)로 가장 높았고, 이밖에 에탐부톨 24.5% (1,261건/5,157명), 이소니아지드 22.2% (1,169건/5,277명), 리팜핀 21.3% (1,121건/5,270명)로 비슷하였다(Table 1). 중증약물이상반응 및 심각한 약물이상반응의 발생률은 리팜핀이 중증 7.4%, 심각함 6.2%로 가장 높았다.
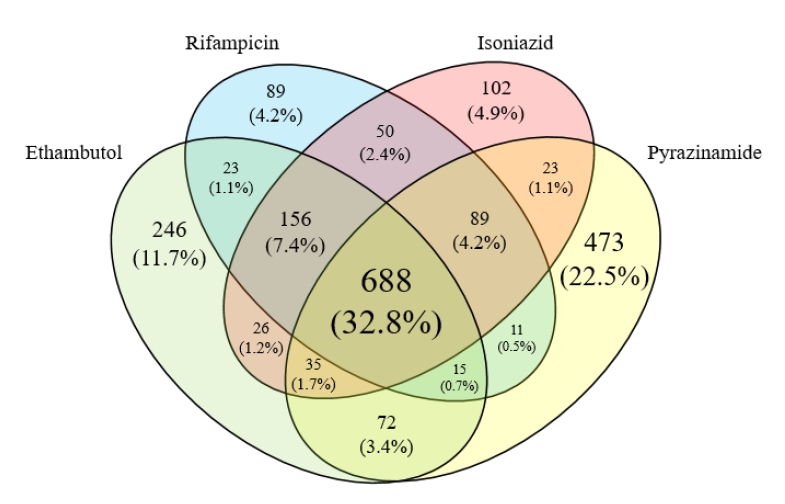
전체 2,098건의 ICSR에서 신고된 원인약제의 개수를 살펴보면 단일 약제를 원인약제로 특정한 경우는 910건(43.4%)에 그쳤으며, 약제별로는 피라진아미드가 473건(52.0%)으로 가장 많았고, 에탐부톨 246건(27.0%), 이소니아지드 102건(11.2%), 리팜핀 89건(9.8%)이었다. 원인약제를 하나로 지목할 수 없었거나 여러 가지 약제가 동시에 원인약제로 의심되어 복수의 약제가 원인약제로 신고된 사례들이 많았는데, 두 가지 약제가 동시에 원인약제로 신고된 경우는 205건(9.8%)이었고, 이 중 에탐부톨과 피라진아미드가 함께 원인약제로 신고된 경우가 72건으로 가장 많았다. 세 가지 약제가 원인 약제로 동시에 신고된 건은 295건(14.1%)으로, 이소니아지드, 리팜핀, 에탐부톨의 조합이 156건으로 가장 많았다. 이밖에 네 약제 모두 원인약제로 신고된 사례도 688건(32.8%)에 달하였다(Fig. 2).
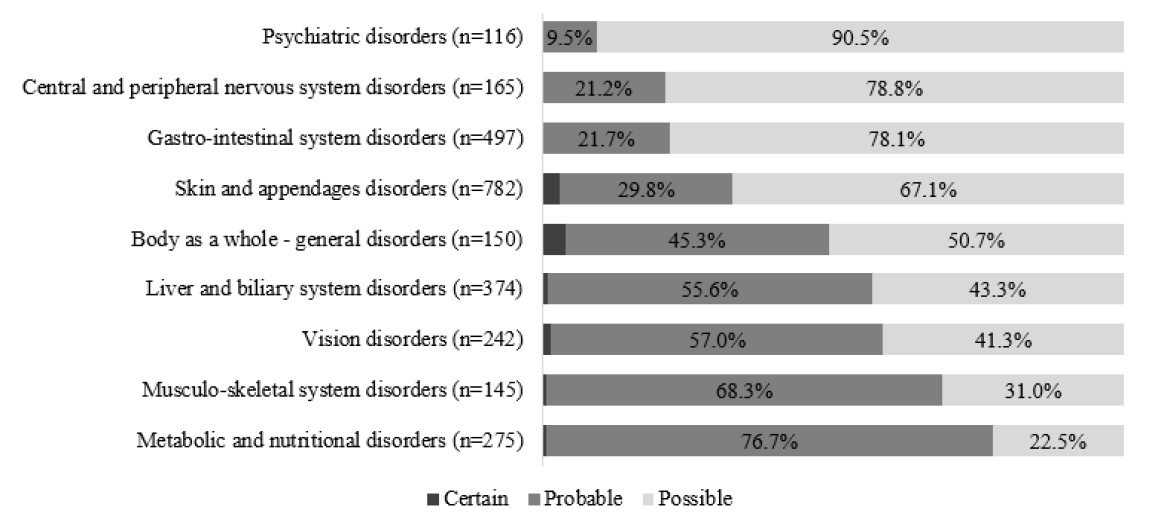
이상소견을 WHO-ART (adverse reaction terminology) 분류 체계에서 정의한 장기 계통별(system-organ class, SOC) 분류에 따라 구분하였을 때[15] 피부와 부속기관 장애가 27.5%로 가장 많았고, 위장관계 장애(17.5%)와 간 및 담도계 질환(13.1%)이 그 뒤를 이었다(Table 2). 피부와 부속기관 장애, 위장관계 장애의 경우 이소니아지드, 리팜핀, 에탐부톨, 피라진아미드 네 가지가 25% 전후로 비슷한 비율로 보고된 반면, 간 및 담도계 질환의 경우 피라진아미드의 비율이 약간 높았고 에탐부톨의 비율이 약간 낮았으며 이소니아지드, 리팜핀의 비율은 비슷하였다. 근골격계 장애와 대사 및 영양 질환의 경우 피라진아미드 관련 이상반응이 각각 56.3%와 71.6%로 가장 많았고, 시각장애의 경우 에탐부톨의 비율이 68.5%로 가장 높았다(Fig. 3). SOC에 따라 약물이상반응의 인과성 평가 또한 차이를 보였는데, 피부와 부속기관 장애와 위장관계 장애의 경우 가능함의 비율이 높았던 반면 근육-골격계 장애, 간 및 담도계 질환, 대사 및 영양 질환과 시각장애의 경우 가능성 높음의 비율이 더 높았다(Fig. 4). 개별증상으로는 가려움증(491건), 간효소증가(260건), 고요산혈증(250건), 시각이상(234건)의 순서로 많이 보고되었다(Table 3).
중증 약물이상반응을 보인 214건 중에서는 간 및 담도계 질환이 46건(21.5%), 피부와 부속기관 장애가 45건(21.0%) 순서로 가장 많이 보고되었다(Table 4). 개별증상으로는 가려움증(21건), 간효소증가(19건), 약물과민반응증후군(19건), 발진(18건), 오심(10건)의 순서로 많이 보고되었다.
심각한 약물이상반응의 경우 전신적 질환이 22.1%, 간 및 담도계 질환이 21.6%, 피부와 부속기관 장애가 15.3%를 차지하였다(Supplementary Table 1). 전신 질환의 경우, 열, 무력증, 피로 등의 증상과 약물과민반응증후군, 아나필락시스반응 등의 과민반응이 있었고, 절반 이상인 22건에서 리팜핀이 원인약제로 보고되었다. 간 및 담도계 질환의 경우 간효소 증가, 간세포 손상, 빌리루빈혈증, 간기능 이상 등의 개별증상이 보고되었으며 이소니아지드, 리팜핀이 각각 26건, 25건 원인약제로 보고되었다. 피부 및 부속기관 장애의 경우 발진, 가려움증, 혈관부종 등의 증상이 있었으며 그중 약 58.7%인 17건에서 리팜핀이 원인약제로 보고되었다.
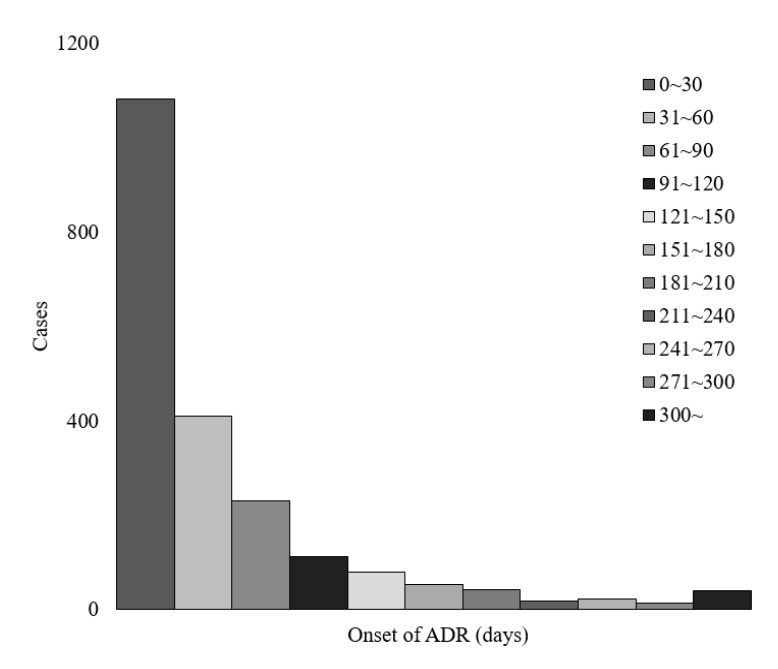
항결핵제 투약 개시 후 이상반응의 발생은 평균 57.0±1.8일(중위수 28일)에 나타났고, 71.1%는 60일 이내에 발생하였다(Fig. 5). SOC별로 구분하였을 때에도 비슷한 양상을 보였으나, 간담도계 질환의 경우 비교적 30일 이내 약물이상반응의 발생 비율이 낮았고(46.0%), 30-60일 사이의 약물이상반응 발생 비율이 높았다(25.7%). 시각장애의 경우 다른 SOC에 비해 발생시점이 길어 평균 88.0 ± 7.1일(중위수 56일)에 증상이 나타났다(Supplementary Fig. 1).
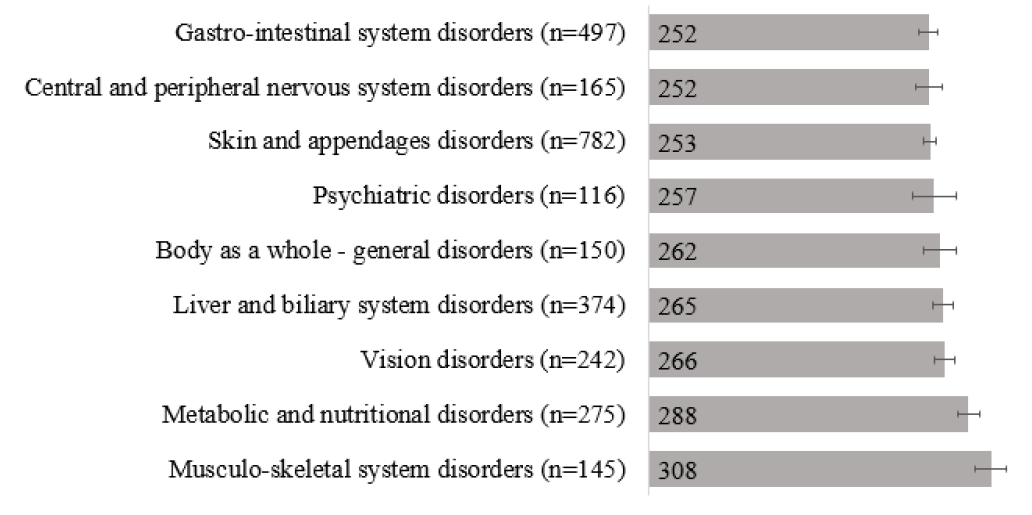
1차 항결핵제 관련 약물이상반응의 발생 여부에 따라 대상 환자를 구분하여 1차 항결핵제의 총 투약일수를 확인한 결과, 약물이상반응이 발생하지 않았던 군은 224.0 ± 3.1일, 약물이상반응이 발생한 군은 247.0 ± 4.7일로 약물이상반응이 발생한 군이 치료 기간이 평균 23일 정도 길었으며, 이는 통계적으로 유의하였다(p = 0.009). 약물이상반응의 심각도가 높아질수록 기간이 길어지는 경향을 보였고, 특히 중증피부유해반응이 발생한 경우 투약일수가 더 증가하였다(Fig. 6). 약물이상반응의 SOC에 따라서도 투약일수에 차이가 있었는데(p = 0.001) 근골격계 장애가 발생한 경우 치료기간이 평균 308.0 ± 13.6일로 가장 길었고, 위장관계 장애와 중추 및 말초신경계 장애의 경우 평균 치료 기간이 각각 252.0 ± 8.0일과 252.0 ± 11.4일로 가장 짧았다(Fig. 7).
10년 동안 보고된 1차 항결핵제의 약물이상반응 중 중증피부유해반응은 총 23명으로 사용량 대비 발생률은 0.4%였다. 23명 모두 약물과민반응증후군(drug reaction with eosinophilia and systemic symptoms, DRESS)으로 진단 받았으며, 그중 한명은 DRESS와 스티븐스존슨증후군(Stevens Johnson syndrome, SJS)/독성표피괴사(toxic epidermal necrolysis, TEN) 중첩반응(SJS/TEN overlap)이 병발한 사례였다. 중증피부유해반응으로 신고된 원인약제는 이소니아지드가 14건으로 가장 많았고 리팜핀 11건, 피라진아미드 10건, 에탐부톨 9건이었다. 23명 중 2명은 중증피부유해반응 발생 후 바로 2차 항결핵제로 약제를 변경하였으며, 나머지 21명은 1차 항결핵제에 대한 탈감작을 시도하였고 16명(76.2%)에서 성공적으로 1차 항결핵제 투여를 완료하였다. 투약을 완료한 환자들의 결핵 치료기간은 평균 306.0 ± 187.6일이었다. 탈감작을 실패한 5명은 2차 항결핵제로 약제를 변경하거나 약제 치료를 중단하고 경과를 관찰하였다.
본 연구에서는 국내 단일기관의 5,482명의 항결핵제 사용자를 대상으로 약물이상반응 모니터링에 따른 이상사례 신고자료를 기반으로 1차 항결핵제의 약물이상반응 발생률과 발생시점, 치료 기간을 분석하였다. 항결핵제에 의한 약물이상반응은 연구 대상의 선정, 모니터링 방법에 따라 그 빈도가 다양하게 나타난다. 1990년 이후 출판된 101개의 결핵약제 약물이상반응 문헌을 정리한 한 종설에서는 1차 항결핵제에 의한 약물이상반응이 8%에서 많게는 85%까지 나타난다고 보고하였다[16]. 영국의 단일기관에서 절반의 인체면역결핍바이러스 감염 환자를 포함한 총 312명의 환자들을 후향적으로 조사한 결과에서는 약물이상반응이 총 104명(33.3%)에서 발생한 것으로 확인되었다[17]. 본 연구에서 확인된 29.3%의 약물이상반응 발생률은 국내 435명의 결핵 환자를 대상으로 약물이상반응을 모니터링한 연구에서 나온 발생률인 52.6%보다는 낮은 수치를 보였는데[5], 특히 해당 연구에서는 피부, 위장관, 간의 이상반응 빈도는 본 연구와 큰 차이가 없었으나 신장과 신경계, 혈액계 이상반응이 높게 보고되는 등 장기별 이상반응의 빈도에 차이를 보였다. 특히 해당 연구에서는 고요산혈증을 신기능이상에 포함시켜서 신장 이상반응의 빈도가 높았는데, 본 연구에서 고요산혈증은 대사 및 영양 질환으로 분류되어 신장 이상반응의 빈도가 높지 않게 나타났다. 이처럼 이상반응의 분류기준 및 입적기준에 따라 빈도는 차이를 보인 것으로 생각된다.
항결핵제는 동시에 여러 가지 약제를 쓰기 때문에 어느 약제가 원인약제인지 감별하기 쉽지 않으며, ICSR에서 신고된 약제가 여러 종류일 경우 정확한 원인약제를 지목할 수 없다는 한계가 있었다. 본 연구에서는 항결핵제 투약 관리의 일환으로 약물이상반응 발생 여부를 모니터링한 자료를 기반으로 하였기 때문에 투약자 전수에 대한 부작용 발생률을 구할 수 있었다. 다만 ICSR이 신고된 시점에서는 약물이상반응이 발생하기 이전의 투약력에 대한 정보밖에 얻을 수 없으며, 이에 대한 인과성을 평가하는 시점에는 해당 약제에 대한 중단 및 증상의 호전, 재투여를 하지 않은 상황인 경우가 많아 인과성 평가에 한계가 있었다. 본 연구에서 ICSR을 후향적으로 분석하였을 때 원인약제가 한 가지로 특정된 경우는 43.4%에 불과하였다. WHO-UMC 인과성 평가 기준에서는 병용약제나 기저 질환 등 약물이상반응을 일으킬 수 있는 다른 원인이 있을 경우 의심약제의 인과성을 상대적으로 낮게 평가하기 때문에 신고된 의심약제가 많을수록 개별 약제에 대한 약물이상반응의 인과성이 상대적으로 약하게 판단된다. 실제 ICSR의 인과성이 가능성 있음으로 평가된 비율은 원인약제가 한 가지인 경우(30.2%)보다 원인약제가 두 가지 이상인 경우(78.5%)에서 월등히 높았다. 원인약제로 네 약제가 모두 신고된 경우는 대부분 네 약제를 모두 투여하고 있던 시점에서 약물이상반응이 발생한 경우로 ICSR 평가 당시 정보에 근거하여 평가하였을 때 네 약제의 인과성 평가 결과가 모두 가능성이 있다고 평가된 경우였다. 네 약제가 원인 약제로 평가된 경우 인과성은 가능성 있음이 80.4%, 가능성 높음이 19.6%였고, 확실함으로 평가된 경우는 없었다. 다만 항결핵제 관련 과민반응의 경우 다약제 과민반응(multidrug hypersensitivity)이 특징적으로 나타나기 때문에 실제로 원인약제가 여러 개였던 사례도 있었을 것으로 추정된다.
항결핵제는 잠복결핵에 이소니아지드가 단독으로 사용되는 경우를 제외하면 단일 약제를 결핵 치료에 사용하였을 때의 약물이상반응에 대한 정보는 드물다[18]. 본 연구에서는 네 가지 항결핵제의 투약 여부와 의심약제 보고를 토대로 약제별 약물이상반응의 발생률을 산출해볼 수 있었다. 그 결과 피라진아미드가 처방 환자수 대비 부작용 발생이 25.1%로 가장 높게 발생하였다. 캐나다에서 10년 동안 수집된 결핵약 투약 환자 430명을 대상으로 한 연구에서도 절대적인 수치는 본 연구보다 낮았으나 피라진아미드의 처방 환자수 대비 약물이상반응 발생률이 5.9%로 가장 높았다[7]. 독일에서 519명의 환자를 대상으로 한 연구에서는 121명이 약물이상 반응으로 원인약제를 최종 중단하였는데, 그중 79건이 피라진아미드, 34건이 이소니아지드, 8건이 리팜핀에 의한 것이었다[19]. 다만 피라진아미드가 유일한 원인약제로 신고된 ICSR 473건 중 202건이 고요산혈증이 포함된 대사 및 영양 질환이었으며 50건이 관절통이 포함된 근골격계 질환이었는데, 대부분이 경증으로 나타나 중증도(5.4%)와 심각도(3.3%)는 다른 약제에 비해 상대적으로 낮았다.
항결핵제에 의한 약물이상반응 발생은 주로 초기 집중 치료기에서 많이 관찰되었다[16]. 중국, 말레이시아, 인도인을 대상으로 한 싱가폴에서의 연구에서도 이소니아지드, 리팜핀, 피라진아미드, 스트렙토마이신을 사용한 뒤 약물이상반응이 발생한 29%의 환자 중 91%가 첫 2달 안에 증상이 발생하였다[20]. 본 연구에서도 첫 약물이상반응 발생 시점이 중위수 28일이었고, 60일 내 약물이상반응의 71.1%가 발생하였다.
한 단일기관에서 10년 동안의 결핵 환자에 대해 약물이상반응 발생을 조사한 결과, 약물이상반응을 경험한 환자의 43.6%가 예상하였던 치료 기간보다 실제 치료 기간이 길어졌다[21]. 본 연구에서도 결핵 진단을 받은 환자를 대상으로 총 치료 기간을 분석한 결과 약물이상반응이 발생하였던 군에서 치료 기간이 23일 정도 길었다. 발생한 약물이상반응의 장기별 분류에서는 근골격계 장애가 발생한 경우 평균 치료 기간이 약 308일로 가장 길었는데, 이는 대부분의 근골격계 장애가 피라진아미드에 의한 관절통이라는 점으로 설명할 수 있다. 피라진아미드는 결핵 초기의 급성 염증성 병변에 효과적으로 작용하기 때문에 6개월의 치료 중 첫 2개월에만 사용하고, 사용하지 못할 경우 이소니아지드, 리팜핀, 에탐부톨 세 약제를 9개월 동안 사용하게 된다[3]. 때문에 피라진아미드에 의해 근골격계 약물이상반응이 발생한 경우 치료 요법의 변경에 따라 치료 기간이 더 길어진 것으로 보인다. 결핵진료지침에 따르면 흔히 발생하는 간독성이나 피부반응, 약제열 등의 경우 약제를 중단한 뒤 재투여해 보거나 다른 약제를 사용하게 되는데[3], 이러한 과정에 의해 치료 기간이 길어질 수 있다. 치료 기간이 길어지면 추가적인 검사 및 외래 방문 등에 의한 경제적 손실도 발생할 수 있다. 발생한 약물이상반응의 종류에 따라 대처방안이 다를 수 있으므로, 환자에 대한 면밀한 관찰 후 적절한 조치를 취하는 것이 중요하다.
본 연구에서 10년 동안 1차 항결핵제에 의한 DRESS 증후군은 23명에게서 확인되어, 발생률은 약 0.4%로 나타났다. 2017년에 이루어진 문헌 분석에 의하면 1차 항결핵제에 의한 DRESS 증후군의 사례가 33예 확인되었다[22]. 일반적으로 중증피부유해반응과 같은 중증 지연성 과민반응에는 원인약제를 재투여하지 않는 것이 원칙이지만, 항결핵제의 경우 약제 변경에 따른 치료효과 감소 우려가 크고 선택할 수 있는 약제가 제한적이라서 탈감작을 시도한 연구 결과들이 보고되어 있다. 태국에서 항결핵제 탈감작을 시행한 25명의 환자를 분석한 연구에서 중증피부유해반응을 겪은 환자의 탈감작 성공률은 62.5%로 나타났고[23], 본 연구에서도 DRESS 증후군 환자에서도 21명 중 16명(76.2%)에게 탈감작이 적용되어 항결핵제 투약을 완료하였다. 탈감작에 실패한 환자 5명 중 한 명은 DRESS 증후군의 간침범에 따른 전격성 간염으로 사망하였고, 다른 한 명은 2차 약제로 치료를 변경하였으며, 나머지 두 명은 더 이상의 항결핵제 투약 없이 치료 중단 후 추적 관찰하였고 한 명은 추적 소실되었다.
본 연구의 한계점은 다음과 같다. 약물이상반응이 신고되면 원내 ICSR 시스템에서 바로 평가 후 데이터베이스에 저장되기 때문에 약제의 중단 및 재투여 이후 경과가 인과성 평가에 반영되지 않았던 사례들이 많았다. 또한 본 연구에서 사용한 약물이상반응 데이터베이스 자료의 특성 상 최종 결핵 치료 결과에 대한 정보는 포함하지 않았다. 항결핵제 이외 외부기관에서 처방한 모든 병용약물에 대한 상세 정보를 포함하지 않아 병용약물에 대한 평가에 일부 제한점이 있었다. 마지막으로 연구 설계 시 1차 항결핵제의 사용 기간에 대한 정보만 수집하여, 2차 약제로 변경하여 치료한 경과에 대해서는 반영이 되지 않아 일부 환자에서 항결핵 치료 기간이 실제보다 저평가 되었을 수 있다. 그러나 본 연구는 한 단일기관에서 장기간 일차 항결핵제를 처방 받은 환자를 대상으로 한 대규모 약물이상반응 자료로써의 의의를 가진다. 또한 대상 선정에 있어 약제를 사용한 모든 환자와 항결핵치료 모니터링을 통해 파악한 모든 보고된 약물이상반응 자료를 대상으로 하였기 때문에, 선택비뚤림이 없는 인구 기반자료로서 추후 결핵 환자의 복약 상담에 있어 부작용 발생에 대한 정보 제공에 활용할 수 있을 것으로 기대된다. 본 연구는 단일 3차 의료기관에서 치료받은 환자를 대상으로 한 만큼 보다 정확한 한국인 일반 인구 집단에서 약물이상반응 프로파일을 구축하기 위해서는 다양한 의료기관을 기반으로 전향적 추적 관찰 연구가 필요하다.
Acknowledgements
This research was supported by a grant from Ministry of Food and Drug Safety to the regional pharmacovigilance center in 2020.
Supplementary Materials
Supplementary Table 1.
System organ class of reported serious adverse drug reactions
Supplementary Figure 1.
Onset of adverse drug reactions according to system organ class.
REFERENCES
1. World Health Organization (WHO). Global tuberculosis report [Internet]. Geneva (CH): WHO, c2019. [cited 2020 Jul 5]. Available from: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-report-2019
2. Korea Centers for Disease Control & Prevention. National tuberculosis program guideline [Internet]. Cheongju (KR): Korea Centers for Disease Control & Prevention, c2020. [cited 2020 Jul 5]. Available from: https://www.kdca.go.kr/board/board.es?mid=a20507020000&bid=0019&act=view&list_no=365688
3. Korea Centers for Disease Control & Prevention. Korean Guidelines for Tuberculosis. 4th ed. Cheongju: Joint Committee for the Revision of Korean Guidelines for Tuberculosis and Korea Centers for Disease Control & Prevention, 2020.
4. Gholami K, Kamali E, Hajiabdolbaghi M, Shalviri G. Evaluation of anti-tuberculosis induced adverse reactions in hospitalized patients. Pharm Pract (Granada) 2006;4:134–138.


5. Kim SH, Lee BH, Lee KD, et al. The prevalence of adverse drug reactions to a short course anti-tuberculosis regimen. Korean J Med 2007;73:496–502.
6. Ormerod LP, Horsfield N. Frequency and type of reactions to antituberculosis drugs: observations in routine treatment. Tuber Lung Dis 1996;77:37–42.


7. Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med 2003;167:1472–1477.


8. Singh A, Prasad R, Balasubramanian V, Gupta N, Gupta P. Prevalence of adverse drug reaction with first-line drugs among patients treated for pulmonary tuberculosis. Clinical Epidemiology and Global Health 2015;3(Suppl 1):S80–S90.

9. Shean K, Streicher E, Pieterson E, et al. Drug-associated adverse events and their relationship with outcomes in patients receiving treatment for extensively drug-resistant tuberculosis in South Africa. PLoS One 2013;8:e63057.



10. Huang YS. Recent progress in genetic variation and risk of antituberculosis drug-induced liver injury. J Chin Med Assoc 2014;77:169–173.


11. Chamorro JG, Castagnino JP, Musella RM, et al. Sex, ethnicity, and slow acetylator profile are the major causes of hepatotoxicity induced by antituberculosis drugs. J Gastroenterol Hepatol 2013;28:323–328.


12. Goldman JL, Sullins A, Sandritter T, Leeder JS, Lowry J. Pediatric pharmacovigilance: enhancing adverse drug reaction reporting in a tertiary care children's hospital. Ther Innov Regul Sci 2013;47:566–571.


13. WHO-UMC. Glossary of pharmacovigilance terms [Internet]. Uppsala: Uppsala Monitoring Centre, c2020. [cited 2020 Jul 5]. Available from: https://www.who-umc.org/global-pharmacovigilance/publications/glossary/
14. WHO-UMC. The use of the WHO-UMC system for standardised case causality assessment [Internet]. Uppsala: Uppsala Monitoring Centre, c2020. [cited 2020 Jul 5]. Available from: https://www.who.int/medicines/areas/quality_safety/safety_efficacy/WHOcausality_assessment.pdf
15. Wallberg M. WHO-ART [Internet]. Uppsala: Uppsala Monitoring Centre, c2009. [cited 2020 Jul 5]. Available from: https://www.who.int/hiv/topics/pharmacovigilance/2_who_art.pdf
16. Prasad R, Singh A, Gupta N. Adverse drug reactions in tuberculosis and management. Indian J Tuberc 2019;66:520–532.


17. Breen RA, Miller RF, Gorsuch T, et al. Adverse events and treatment interruption in tuberculosis patients with and without HIV co-infection. Thorax 2006;61:791–794.



18. Forget EJ, Menzies D. Adverse reactions to first-line antituberculosis drugs. Expert Opin Drug Saf 2006;5:231–249.


19. Schaberg T, Rebhan K, Lode H. Risk factors for side-effects of isoniazid, rifampin and pyrazinamide in patients hospitalized for pulmonary tuberculosis. Eur Respir J 1996;9:2026–2030.


20. Clinical trial of six-month and four-month regimens of chemotherapy in the treatment of pulmonary tuberculosis. Am Rev Respir Dis 1979;119:579–585.

21. Castro AT, Mendes M, Freitas S, Roxo PC. Incidence and risk factors of major toxicity associated to first-line antituberculosis drugs for latent and active tuberculosis during a period of 10 years. Rev Port Pneumol (2006) 2015;21:144–150.


Suspected drug distribution in ICSRs according to system organ class. ICSR, individual case safety report.

Figure 3.
Total treatment period according to the seriousness of adverse drug reaction. ADR, adverse drug reaction; SCAR, severe cutaneous adverse reactions.

Figure 6.
Table 1.
Summary of ICSRs according to drug
Table 2.
System organ class of reported adverse drug reactions
Table 3.
Top 30 most frequently reported symptoms of adverse drug reactions
Table 4.
System organ class of reported severe adverse drug reactions
-
METRICS

-
- 1 Crossref
- 0 Scopus
- 3,330 View
- 110 Download




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



