 |
 |
| Korean J Med > Volume 96(5); 2021 > Article |
|
Abstract
The most important thing for the management of drug susceptible pulmonary tuberculosis is to diagnose active pulmonary tuberculosis as soon as possible and prevent the occurrence of new patients through appropriate treatment. Therefore, it should be a priority to quickly detect tuberculosis mycobacterium and quickly exclude drug-resistant tuberculosis before treatment begins. To this end, recent guidelines recommend the general use of Mycobacterium tuberculosis (MTB) polymerase chain reaction (PCR) tests, Xpert MTB/RIF tests, and rapid sensitivity tests through line probe assay (LPA). In addition, if the results of the test are positive, it is important to establish an in-hospital reporting system so that rapid reporting can be made. The treatment principle for drug susceptible pulmonary tuberculosis is 2 months of initial intensive phase (isoniazid, rifampin, ethambutol, pyrazinamide) followed by 4 months of maintenance phase (isoniazid, rifampin). Despite global efforts to shorten the duration of the treatment, the treatment of drug susceptible pulmonary tuberculosis has not changed for more than 35 years, and problems such as increased side effects and reduced drug adherence are serious obstacles to tuberculosis management. Therefore, efforts have been steadily made to shorten the treatment period through the combination of new drugs worldwide, and after many failures, they are finally paying off. A recently published Study 31/A5349 study found that 4 months short-term regimen using rifapentine (RPT) and moxifloxacin (MFX) demonstrated non-inferiority in existing standard regimen, as the result, a revision of World Health Organization guidelines is scheduled that 4 months short-term regimen using RPT and MFX may be an alternative. However, it is unlikely that RPT/MFX 4 months short-term regimen will be applied immediately in Korea because the use of RPT is currently limited in Korea due to the high frequency of side effects.
мөңк·ј лӢӨм ңлӮҙм„ұ кІ°н•өмқҳ 진лӢЁкіј м№ҳлЈҢлҠ” л§Өмҡ° л№ лҘё мҶҚлҸ„лЎң л°ңм „н•ҙмҷ”кі , к·ём—җ л”°лқј 진лЈҢм§Җм№Ёмқҙ мҲҳм°ЁлЎҖ к°ңм •лҗҳм—Ҳмңјл©°, мһ ліөкІ°н•ө л°Ҹ 비결н•ө н•ӯмӮ°к· нҸҗм§Ҳнҷҳмқҳ 진лӢЁ л°Ҹ м№ҳлЈҢ м—ӯмӢң л§ҺмқҖ ліҖнҷ”к°Җ мһҲм—ҲлӢӨ. к·ёлҹ¬лӮҳ к°җмҲҳм„ұ кІ°н•өмқҳ 1м°Ё м№ҳлЈҢмқё 4м ң лӢЁкё°мҡ”лІ•(isoniazid [INH], rifampicin [RIF], ethambutol [EMB]кіј pyrazinamide [PZA])мқҖ кіјкұ° 35л…„ лҸҷм•Ҳ ліҖн•ҳм§Җ м•Ҡм•ҳкі , м№ҳлЈҢ кё°к°„мқҖ м—¬м „нһҲ мөңмҶҢ 6к°ңмӣ”мқҙ кұёлҰ°лӢӨ. мқҙл Үл“Ҝ кёҙ м№ҳлЈҢмҡ”лІ•мқҖ кІ°н•ө кҙҖлҰ¬м—җ мһҲм–ҙ мӢ¬к°Ғн•ң мһҘм• л¬јмқҙкё° л•Ңл¬ём—җ м„ёкі„м ҒмңјлЎң мқҙ м№ҳлЈҢ кё°к°„мқ„ лӢЁм¶•мӢңнӮӨл ӨлҠ” л…ёл Ҙмқҙ кҫёмӨҖнһҲ 진н–үлҗҳм–ҙ мҷ”мңјл©°, мөңк·јм—җ л“ңл””м–ҙ к·ё кІ°мӢӨмқ„ ліҙмқҙкі мһҲлӢӨ. мқҙ кёҖм—җм„ңлҠ” к°җмҲҳм„ұ кІ°н•ө 진лӢЁмқҳ мөңмӢ м§ҖкІ¬, нҠ№нһҲ мӨ‘н•©нҡЁмҶҢм—°мҮ„л°ҳмқ‘(polymerase chain reaction, PCR) кІҖмӮ¬ л°Ҹ мӢ мҶҚ к°җмҲҳм„ұ кІҖмӮ¬ мӮ¬мҡ©мқҳ мөңк·ј м§Җм№Ё, м•Ҫм ң к°җмҲҳм„ұ нҸҗкІ°н•ө м№ҳлЈҢмқҳ м—ӯмӮ¬ л°Ҹ м№ҳлЈҢ кё°к°„мқ„ лӢЁм¶•мӢңнӮӨкё° мң„н•ҙ мқҙлҜё мҷ„лЈҢлҗҳм—Ҳкұ°лӮҳ нҳ„мһ¬ 진н–ү мӨ‘мқё мһ„мғҒ м—°кө¬, мқҙлҘј к·јкұ°лЎң н•ң м„ёкі„ліҙкұҙкё°кө¬(World Health Organization, WHO) м§Җм№Ё к°ңм •кіј к°ҷмқҖ мөңмӢ м§ҖкІ¬мқ„ мӮҙнҺҙліҙкі мһҗ н•ңлӢӨ. нҸҗмҷё кІ°н•ө л°Ҹ м•Ҫм ң лӮҙм„ұкІ°н•өмқҳ 진лӢЁкіј м№ҳлЈҢлҠ” ліёкі м—җм„ң м ңмҷён•ҳмҳҖлӢӨ.
кІ°н•ө кҙҖлҰ¬лҘј мң„н•ҙ к°ҖмһҘ мӨ‘мҡ”н•ң кІғмқҖ нҷңлҸҷм„ұ нҸҗкІ°н•ө нҷҳмһҗлҘј к°ҖлҠҘн•ң н•ң л№ЁлҰ¬ 진лӢЁн•ҳкі м Ғм Ҳн•ң м№ҳлЈҢлҘј нҶөн•ҙ кІ°н•өк· м „нҢҢлЎң мқён•ң мғҲлЎңмҡҙ нҷҳмһҗмқҳ л°ңмғқмқ„ л§үлҠ” кІғмқҙлӢӨ. к·ёлҹ¬лӮҳ кІ°н•өмқҖ лӢӨм–‘н•ң мһ„мғҒ мҶҢкІ¬мқ„ ліҙмқҙл©° нқүл¶Җ л°©мӮ¬м„ кІҖмӮ¬л§ҢмңјлЎң нҷ•м§„мқҙ нһҳл“Өкі , кІҖмІҙлҘј м–»мқ„ мҲҳ м—ҶлҠ” кІҪмҡ°лҸ„ л§Һмқ„ лҝҗлҚ”лҹ¬ 진лӢЁмқ„ мң„н•ҙ м „нҶөм ҒмңјлЎң мӢңн–үлҗҳм–ҙ мҳЁ н•ӯмӮ°к· лҸ„л§җ кІҖмӮ¬мқҳ кІҪмҡ° мөңк·ј л№ҲлҸ„к°Җ мҰқк°Җ 추세мқё 비결н•өн•ӯмӮ°к· кіј к°җлі„н• мҲҳ м—Ҷм–ҙ лҜјк°җлҸ„к°Җ л–Ём–ҙ진лӢӨ[1,2]. нҷ•м§„мқ„ мң„н•ң н•ӯмӮ°к· л°°м–‘ кІҖмӮ¬мқҳ кІҪмҡ° мһҗлҸҷнҷ” м•ЎмІҙл°°м§Җ(BACTEC460)лҘј лҸҷмӢңм—җ мӮ¬мҡ©н•ҳм—¬лҸ„ 2-6мЈј м •лҸ„мқҳ кёҙ мӢңк°„мқҙ мҶҢмҡ”лҗҳкё°[3] л•Ңл¬ём—җ 진лӢЁмқҙ лҠҰм–ҙм§ҖлҠ” кІҪмҡ°к°Җ л§ҺлӢӨлҠ” кІғлҸ„ л¬ём ңмқҙлӢӨ. мөңк·јм—җ н•ӯмӮ°к· лҸ„л§җ кІҖмӮ¬ліҙлӢӨ лҜјк°җлҸ„к°Җ лҶ’мңјл©ҙм„ң кё°мЎҙмқҳ л°°м–‘ кІҖмӮ¬ліҙлӢӨ л№ лҘҙкІҢ кІ°н•өмқ„ нҷ•м§„н• мҲҳ мһҲмңјл©°, м•Ҫм ңлӮҙм„ұ м—¬л¶ҖлҘј н•Ёк»ҳ нҸүк°Җн• мҲҳ мһҲлҠ” м—¬лҹ¬ к°Җм§Җ кІҖмӮ¬лІ•мқҙ к°ңл°ңлҗҳм–ҙ мһ„мғҒм Ғ мӨ‘мҡ”м„ұмқҙ мҰқк°Җн•ҳкі мһҲлӢӨ.
кІ°н•өк· л¶„мһҗ 진лӢЁ кІҖмӮ¬лҠ” кІ°н•өк· м—җл§Ң нҠ№мқҙн•ҳкІҢ мЎҙмһ¬н•ҳлҠ” DNAлӮҳ RNAмқҳ нҠ№м • м—јкё°м„ңм—ҙмқ„ PCRмқ„ мқҙмҡ©н•ҳм—¬ мҰқнҸӯн•ҳм—¬ нҷ•мқён•ҳлҠ” кІҖмӮ¬мқҙлӢӨ. 분мһҗ 진лӢЁ кІҖмӮ¬лҠ” н•ӯмӮ°к· лҸ„л§җ л°Ҹ л°°м–‘кІҖмӮ¬м—җ 비н•ҙ 비мҡ©мқҙ л§Һмқҙ л“Өм§Җл§Ң, лҜјк°җлҸ„мҷҖ нҠ№мқҙлҸ„к°Җ лҶ’кі , кІ°кіјлҘј м–»кё°к№Ңм§Җмқҳ мӢңк°„мқҙ 24-48мӢңк°„мңјлЎң 짧мқҖ мһҘм җмқҙ мһҲлӢӨ[4]. кІ°н•өк· л¶„мһҗ 진лӢЁ кІҖмӮ¬м—җлҠ” лӢӨм–‘н•ң л°©лІ•мқҙ мһҲмңјл©° м§ҖлӮң 10м—¬л…„к°„ мғҲлЎңмҡҙ 분мһҗ 진лӢЁ кІҖмӮ¬к°Җ л§Һмқҙ к°ңл°ңлҗҳм—Ҳмңјл©° PCRмқ„ нҶөн•ң кІ°н•өк· н•өмӮ° мҰқнҸӯ кІҖмӮ¬(nucleic acid amplification tests, NAATs)лҠ” кІ°н•өк· мқ„ нҷ•мқён•ҳлҠ” кё°лҠҘ мҷём—җлҸ„ кІ°н•ө м№ҳлЈҢм—җ н•өмӢ¬мқҙ лҗҳлҠ” м•Ҫм ңл“Өм—җ лҢҖн•ң лӮҙм„ұ м—¬л¶Җк№Ңм§Җ м „нҶөм Ғ м•Ҫм ң к°җмҲҳм„ұ кІҖмӮ¬ліҙлӢӨ л№ лҘҙкІҢ нҷ•мқён• мҲҳ мһҲлӢӨ[5]. нҳ„мһ¬к№Ңм§Җ WHOм—җм„ң мҠ№мқёлҗң NAATsм—җлҠ” line probe assay (LPA), Xpert MTB/RIF, loop-mediated isothermal amplification (LAMP), Truelab л“ұмқҙ мһҲмңјлӮҳ[5] көӯлӮҙм—җм„ңлҠ” LAMP, Truelabм—җ лҢҖн•ң нҸүк°ҖмҷҖ к·јкұ°к°Җ л¶ҖмЎұн•ҳлӢӨ. көӯлӮҙ кІ°н•ө 진лЈҢм§Җм№Ём—җм„ң 2011л…„(мҙҲнҢҗ)м—җлҠ” 1) нҸҗкІ°н•өмқҙ мқҳмӢ¬лҗҳлӮҳ лҸ„л§җ кІҖмӮ¬к°Җ мқҢм„ұмқј л•Ң нҳ№мқҖ 2) н•ӯмӮ°к· лҸ„л§җ кІҖмӮ¬к°Җ м–‘м„ұмқҙм§Җл§Ң 비결н•ө н•ӯмӮ°к· мқҳ к°ҖлҠҘм„ұмқҙ мһҲмқ„ л•Ң мӢӨмӢңн• кІғмқ„ к¶Ңкі н•ҳмҳҖлӢӨ[6]. н•ҳм§Җл§Ң 2017л…„(3нҢҗ)л¶Җн„° кІ°н•өмқҙ мқҳмӢ¬лҗ л•Ң н•ӯмӮ°к· лҸ„л§җ л°Ҹ л°°м–‘ кІҖмӮ¬мҷҖ н•Ёк»ҳ PCR кІҖмӮ¬лҘј л°ҳл“ңмӢң мӢңн–үмқ„ н•ҙм•ј н•ңлӢӨкі лӘ…мӢңн•ҳкі мһҲлӢӨ[7]. көӯлӮҙм—җм„ңлҠ” лҜјк°җлҸ„мҷҖ нҠ№мқҙлҸ„к°Җ лҶ’мқҖ вҖҳмӢӨмӢңк°„ мӨ‘н•© нҡЁмҶҢ м—°мҮ„ л°ҳмқ‘(real-time PCR)вҖҷмқҙ мЈјлЎң мӮ¬мҡ©лҗҳкі мһҲмңјл©° мқҙлҘј мӮ¬мҡ©н•ң л°©лІ•м—җлҠ” нҶөмғҒм Ғмқё л°©лІ•кіј Xpert MTB/RIF кІҖмӮ¬лІ•(Xpert)мқҙ мһҲлӢӨ.
XpertлҠ” мһҗлҸҷнҷ”лҗң real-time PCR кІҖмӮ¬лЎң мҲҷл Ёлҗң кІҖмӮ¬мһҗк°Җ м•„лӢҲлҚ”лқјлҸ„ кІҖмІҙлҘј л°”лЎң кІҖмӮ¬ нӮӨнҠём—җ м Ғмҡ©н•ҳкё° л•Ңл¬ём—җ кІҖмӮ¬ кіјм •мқҙ лӢЁмҲңн•ҳл©° м•Ҫ 2мӢңк°„ лӮҙм—җ кІ°кіјлҘј нҷ•мқён• мҲҳ мһҲлҠ” мһҘм җмқҙ мһҲмңјлӮҳ нҶөмғҒм Ғмқё real-time PCRм—җ 비н•ҳм—¬ кІҖмӮ¬ нӮӨнҠёмҷҖ мһҘ비к°Җ кі к°ҖлқјлҠ” лӢЁм җмқҙ мһҲлӢӨ[8]. н•ҳм§Җл§Ң XpertлҠ” кІ°н•өк· мқҳ мЎҙмһ¬ нҷ•мқёкіј лҸҷмӢңм—җ RIF лӮҙм„ұ м—¬л¶ҖлҘј к°ҷмқҙ кІҖмӮ¬н• мҲҳ мһҲм–ҙ мӢ мҶҚн•ң кІ°н•өмқҳ 진лӢЁ л°Ҹ RIF лӮҙм„ұ кІ°н•өмқҳ 진лӢЁмқҙ лӘЁл‘җ к°ҖлҠҘн•ҳм—¬ мөңк·ј көӯлӮҙ л§ҺмқҖ лі‘мӣҗм—җм„ң мӮ¬мҡ©лҗҳкі мһҲлӢӨ.
көӯлӮҙ кІ°н•ө 진лЈҢм§Җм№Ём—җм„ңлҠ” 2014л…„(2нҢҗ)л¶Җн„° Xpert кІҖмӮ¬м—җ лҢҖн•ҙ 1) мһ¬м№ҳлЈҢмҷҖ к°ҷмқҙ лӢӨм ңлӮҙм„ұкІ°н•өмқҳ к°ҖлҠҘм„ұмқҙ лҶ’мқҖ кІҪмҡ° нҳ№мқҖ 2) мӨ‘мҰқ кІ°н•өмқҙлӮҳ мқёмІҙл©ҙм—ӯкІ°н•Қл°”мқҙлҹ¬мҠӨ к°җм—јмқём—җм„ң л°ңмғқн•ң кІ°н•өмІҳлҹј м•Ҫм ңлӮҙм„ұ м—¬л¶ҖлҘј мӢ мҶҚн•ҳкІҢ нҷ•мқён•ҙм•ј н•ҳлҠ” кІҪмҡ° нҳ№мқҖ 3) к·ё мҷё мғҒнҷ©м—җм„ң лӢӨм ңлӮҙм„ұ кІ°н•өмқ„ кІҖм¶ңн•ҳкё° мң„н•ң лӘ©м ҒмңјлЎң мӢӨмӢңн• мҲҳ мһҲлӢӨкі к¶Ңкі н•ҳмҳҖлӢӨ[9]. н•ҳм§Җл§Ң 2020л…„м—җ к°ңм •лҗң 4нҢҗм—җм„ңлҠ” 1) лӢӨм ңлӮҙм„ұ кІ°н•өмқҙ мқҳмӢ¬лҗҳлҠ” кІҪмҡ° нҳ№мқҖ 2) мӢ мҶҚн•ҳкІҢ лӮҙм„ұ м—¬л¶ҖлҘј нҷ•мқён•ҙм•ј н•ҳлҠ” кІҪмҡ° л°ҳл“ңмӢң мӢңн–үн•ҙм•ј н•ңлӢӨкі лӘ…мӢңн•ҳмҳҖкі , 3) мӢ мҶҚн•ң кІ°н•ө 진лӢЁмқҙ н•„мҡ”н•ң кІҪмҡ° мӢңн–үн• мҲҳ мһҲлӢӨкі к¶Ңкі н•ҳкі мһҲлӢӨ[10]. 2014л…„м—җ л°ңн‘ңлҗң Xpertмқҳ 진лӢЁ м •нҷ•лҸ„м—җ лҢҖн•ң мІҙкі„м Ғ л¬ён—Ңкі м°° кІ°кіј, лҸ„л§җ л°Ҹ л°°м–‘м–‘м„ұ кІҖмІҙм—җм„ң лҜјк°җлҸ„к°Җ 98%мҳҖкі лҸ„л§җ мқҢм„ұ л°Ҹ л°°м–‘ м–‘м„ұкІҖмІҙм—җм„ң лҜјк°җлҸ„лҠ” 67%лЎң нҷ•мқёлҗҳм—ҲлӢӨ[11]. мқҙлҹ¬н•ң лҸ„л§җ мқҢм„ұ кІҖмІҙм—җм„ңмқҳ лӮ®мқҖ лҜјк°җлҸ„лҘј н–ҘмғҒмӢңнӮӨкё° мң„н•ҙ Xpert MTB/RIF ultraк°Җ к°ңл°ңлҗҳм—Ҳмңјл©° 2017л…„л¶Җн„° WHOм—җ мҠ№мқёмқ„ л°ӣм•ҳкі , 2021л…„м—җ л°ңн‘ңлҗң Xpert MTB/RIF ultraмқҳ 진лӢЁ м •нҷ•лҸ„м—җ кҙҖн•ң мІҙкі„м Ғ л¬ён—Ңкі м°° кІ°кіј кё°мЎҙмқҳ XpertмҷҖ 비көҗн•ҳмҳҖмқ„ л•Ң нҸҗкІ°н•ө 진лӢЁмқҳ лҜјк°җлҸ„ л°Ҹ нҠ№мқҙлҸ„(95% мӢ лў°кө¬к°„)лҠ” Xpert MTB/RIF Ultraм—җм„ң 90.9% (86.2-94.7), 95.6% (93.0-97.4)мҳҖкі , Xpertм—җм„ң 84.7% (78.6-89.9), 98.4% (97.0-99.3)лЎң лҜјк°җлҸ„лҠ” 6.2% н–ҘмғҒлҗҳм—ҲмңјлӮҳ нҠ№мқҙлҸ„к°Җ 2.8% к°җмҶҢн•ң кІ°кіјлҘј ліҙмҳҖлӢӨ. лҸ„л§җ мқҢм„ұ л°Ҹ л°°м–‘ м–‘м„ұ кІҖмІҙм—җм„ң 진лӢЁ лҜјк°җлҸ„лҠ” Xpert MTB/RIF ultraм—җм„ң 77.5%лЎң кё°мЎҙмқҳ Xpertмқҳ 60.6%ліҙлӢӨ 16.9%лЎң мӣ”л“ұн•ң мҰқк°ҖлҘј ліҙмҳҖлӢӨ[12]. мөңк·јм—җлҠ” RIFлҝҗл§Ң м•„лӢҲлқј fluoroquinolone (FQ) л°Ҹ мЈјмӮ¬м ңм—җ лҢҖн•ң лӮҙм„ұк№Ңм§Җ нҷ•мқён• мҲҳ мһҲлҠ” Xpert XDRмқҙ к°ңл°ңлҗҳм—ҲлӢӨ.
кІ°н•ө кҙҖлҰ¬м—җм„ң л№ лҘё 진лӢЁкіј н•Ёк»ҳ мӨ‘мҡ”н•ң мқҙмҠҲлҠ” м•Ҫм ңлӮҙм„ұмқҙлӢӨ. ліёкі м—җм„ңлҠ” м•Ҫм ң к°җмҲҳм„ұ нҸҗкІ°н•өмқ„ мЈјлЎң лӢӨлЈЁкі мһҲмңјлӮҳ м•Ҫм ң к°җмҲҳм„ұ нҸҗкІ°н•өмқ„ м№ҳлЈҢн•ҳкё° мң„н•ҙм„ңлҠ” м№ҳлЈҢ мӢңмһ‘ м „м—җ м•Ҫм ң лӮҙм„ұкІ°н•өмқ„ л°°м ңн•ҳлҠ” кІғмқҙ мҡ°м„ м ҒмңјлЎң мқҙлЈЁм–ҙм ём•ј н•ңлӢӨ. м „нҶөм ҒмңјлЎң н•ӯкІ°н•ө м•Ҫм ң к°җмҲҳм„ұ кІҖмӮ¬лҠ” кі мІҙ нҳ№мқҖ м•ЎмІҙл°°м§ҖлҘј мӮ¬мҡ©н•ҳм—¬ нҠ№м • н•ӯкІ°н•өм•Ҫм ңлҘј нҸ¬н•Ён•ң л°°м§Җм—җ к· мқ„ м ‘мў…н•ң л’Ө л°°м–‘ м—¬л¶ҖлҘј кҙҖм°°н•ҳлҠ” л°©лІ•мңјлЎң, кі мІҙл°°м§ҖлҘј мӮ¬мҡ©н•ҳлҠ” кІҪмҡ° кІ°кіј ліҙкі к№Ңм§Җ м•Ҫ 3к°ңмӣ” мқҙмғҒ мҶҢмҡ”лҗҳлҠ” лӢЁм җмқҙ мһҲлӢӨ. л”°лқјм„ң лӮҙм„ұм—¬л¶ҖлҘј мӢ мҶҚн•ҳкІҢ нҷ•мқён•ҳкі мһҗ н•ӯкІ°н•өм•Ҫм ңмқҳ лӮҙм„ұм—җ кҙҖм—¬н•ҳлҠ” нҠ№м • мң м „мһҗ лҸҢм—°ліҖмқҙлҘј нҷ•мқён•ҳлҠ” 분мһҗмғқл¬јн•ҷм Ғ л°©лІ•мқҙ лҸ„мһ…лҗҳм—Ҳмңјл©°, RIFмҷҖ INHм—җ лҢҖн•ң мӢ мҶҚ к°җмҲҳм„ұ кІҖмӮ¬мқё LPAк°Җ ліҙнҺёнҷ”лҗҳм–ҙ лӢӨм ңлӮҙм„ұ кІ°н•өмқҳ 진лӢЁм—җ мқҙмҡ©лҗҳкі мһҲлӢӨ. LPAлҠ” лҸ„л§җм–‘м„ұмқё кІҖмІҙмҷҖ л°°м–‘лҗң к· мЈј лӘЁл‘җлЎң мӢңн–үн• мҲҳ мһҲмңјл©° 2-3мқј лӮҙм—җ кІ°кіј нҷ•мқёмқҙ к°ҖлҠҘн•ҳлӢӨ. көӯлӮҙм—җм„ң мӮ¬мҡ© мӨ‘мқё кІҖмӮ¬лІ•мқҖ GenoType MTBDRplus (Hain Lifesciences-Bruker, Nehren, Germany)мқҙл©° RIF лӮҙм„ұмқҳ 진лӢЁмқҖ rpoB мң м „мһҗмқҳ лҸҢм—°ліҖмқҙлҘј, INH лӮҙм„ұмқҳ 진лӢЁмқҖ katG, inhA мң м „мһҗмқҳ лҸҢм—°ліҖмқҙлҘј кІҖм¶ңн•ҳм—¬ лӮҙм„ұ м—¬л¶ҖлҘј 진лӢЁн•ңлӢӨ[13]. л©”нғҖ분м„қ кІ°кіј INH лӮҙм„ұ, RIF лӮҙм„ұ, лӢӨм ңлӮҙм„ұ кІ°н•өмқҳ 진лӢЁ лҜјк°җлҸ„лҠ” 91%, 96%, 91%мқҙкі , нҠ№мқҙлҸ„лҠ” 99%, 98%, 99%мҳҖлӢӨ[14]. мөңк·јм—җлҠ” GenoType MTBDRslмқҙ к°ңл°ңлҗҳм–ҙ INHмҷҖ RIF лҝҗл§Ң м•„лӢҲлқј FQ, EMB, kanamycin, amikacinм—җ лҢҖн•ң лӮҙм„ұмқ„ нҷ•мқён•ҳм—¬ кі л¬ҙм Ғмқё кІ°кіјлҘј ліҙм—¬мЈјкі мһҲлӢӨ[15].
көӯлӮҙ кІ°н•ө진лЈҢм§Җм№Ём—җм„ңлҠ” 2011л…„(мҙҲнҢҗ)л¶Җн„° 1) лӢӨм ңлӮҙм„ұкІ°н•өмқҙ мқҳмӢ¬лҗҳлҠ” кІҪмҡ° нҳ№мқҖ 2) мһ¬м№ҳлЈҢ мӢңм—җ мӢ мҶҚ лӮҙм„ұ кІҖмӮ¬мӢңн–үмқ„ к¶Ңкі н•ҳмҳҖмңјл©°, 2014л…„(2нҢҗ)л¶Җн„°лҠ” 1) мһ¬м№ҳлЈҢмҷҖ к°ҷмқҙ лӢӨм ңлӮҙм„ұ кІ°н•өмқҳ к°ҖлҠҘм„ұмқҙ лҶ’мқҖ кІҪмҡ° лҸ„л§җ м–‘м„ұ кІҖмІҙ нҳ№мқҖ л°°м–‘лҗң кІ°н•өк· мЈјлҘј лҢҖмғҒмңјлЎң мӢ мҶҚ лӮҙм„ұ кІҖмӮ¬ мӢңн–үмқ„ к¶Ңкі н•ҳмҳҖлӢӨ[6]. к·ёлҹ¬лӮҳ 2017л…„(3нҢҗ) мқҙнӣ„ 1) мһ¬м№ҳлЈҢ л“ұ лӢӨм ңлӮҙм„ұ кІ°н•өмқҙ мқҳмӢ¬лҗҳлҠ” кІҪмҡ° лҸ„л§җ м–‘м„ұ кІҖмІҙ нҳ№мқҖ л°°м–‘лҗң кІ°н•өк· мЈјлҘј лҢҖмғҒмңјлЎң мӢ мҶҚ лӮҙм„ұ кІҖмӮ¬лҘј л°ҳл“ңмӢң мӢңн–үн•ҙм•ј н•ңлӢӨкі лӘ…мӢңн•ҳмҳҖкі [7], 2020л…„(4нҢҗ)л¶Җн„°лҠ” 1) лӘЁл“ кІ°н•ө нҷҳмһҗмқҳ мІ« л°°м–‘к· мЈјм—җ лҢҖн•ҙ мӢ мҶҚ к°җмҲҳм„ұ кІҖмӮ¬мҷҖ нҶөмғҒ к°җмҲҳм„ұ кІҖмӮ¬лҘј н•Ёк»ҳ мӢңн–үн•ҙм•ј н•ҳл©°, 2) н•ӯмӮ°к· лҸ„л§җ м–‘м„ұмқё кІҖмІҙлҘј мқҙмҡ©н•ҳм—¬ мӢ мҶҚ к°җмҲҳм„ұ кІҖмӮ¬лҘј мӢңн–үн• мҲҳ мһҲмңјл©°, 3) INH лҳҗлҠ” RIFм—җ лӮҙм„ұмқҙ кІҖм¶ңлҗң кІҪмҡ° FQмқ„ нҸ¬н•Ён•ң мқҙм°Ё н•ӯкІ°н•өм ңм—җ лҢҖн•ң мӢ мҶҚ к°җмҲҳм„ұ кІҖмӮ¬мҷҖ нҶөмғҒ к°җмҲҳм„ұ кІҖмӮ¬лҘј н•Ёк»ҳ мӢңн–үн•ҙм•ј н•ңлӢӨкі к°ңм •к¶Ңкі н•ҳмҳҖлӢӨ[10].
нҸҗкІ°н•өмқҳ л№ лҘё 진лӢЁкіј м№ҳлЈҢлҘј мң„н•ҙм„ңлҠ” мң„м—җм„ң м–ёкёүн•ң мӢ мҶҚн•ң кІҖмӮ¬ л°©лІ• мһҗмІҙлҸ„ мӨ‘мҡ”н•ҳм§Җл§Ң к·ё кІҖмӮ¬ кІ°кіјк°Җ м–‘м„ұмқј л•Ң мһ„мғҒмқҳк°Җ л№ лҘҙкі м Ғм Ҳн•ң мІҳм№ҳлҘј н• мҲҳ мһҲлҸ„лЎқ мӢ мҶҚн•ң ліҙкі мІҙкі„лҘј 갖추лҠ” кІғ м—ӯмӢң л§Өмҡ° мӨ‘мҡ”н•ҳлӢӨ. кІ°н•өмқҳ 진лӢЁ л°Ҹ м№ҳлЈҢмқҳ м§Җм—°мқҖ нҷҳмһҗмқҳ мӮ¬л§қлҘ мқ„ мҰқк°ҖмӢңнӮӨкі , кІ°н•өк· мқҳ м „нҢҢлҘј мҰқк°ҖмӢңнӮ¬ мҲҳ мһҲлӢӨ. нҠ№нһҲ л°ҖнҸҗлҗң кіөк°„м—җм„ң нҷҳмһҗл“Өмқҙ 집лӢЁмғқнҷңмқ„ н•ҳкі мһҲлҠ” мқҳлЈҢкё°кҙҖм—җм„ң кіөкё° л§Өк°ңм„ұ м „м—јлі‘мқё нҸҗкІ°н•ө нҷҳмһҗк°Җ л°ңмғқн• кІҪмҡ°, мЈјмң„ нҷҳмһҗм—җкІҢ кІ°н•өк· мқҙ к°җм—јлҗҳкё° мү¬мҡ°л©° нҷңлҸҷм„ұ кІ°н•өмңјлЎң 진н–үн• мң„н—ҳм„ұ л°Ҹ мӨ‘мҰқ кІ°н•өмқҙ л°ңмғқн• мң„н—ҳм„ұлҸ„ мҰқк°Җн•ңлӢӨ. кІ°н•өмқҳ 진лӢЁ л°Ҹ м№ҳлЈҢк°Җ м§Җм—°лҗҳлҠ” мӣҗмқёмқҖ нҒ¬кІҢ нҷҳмһҗ мҲҳ진 м§Җм—°кіј мқҳмӮ¬ 진лӢЁ м§Җм—°мңјлЎң кө¬л¶„н• мҲҳ мһҲлҠ”лҚ°, мқҳмӮ¬ 진лӢЁ м§Җм—°мқҖ 1) нҷҳмһҗк°Җ лӮҙмӣҗн•ң л’Ө кІ°н•ө кІҖмӮ¬ мӢңн–үк№Ңм§Җ, 2) кІ°н•ө кІҖмӮ¬ нӣ„ 진лӢЁк№Ңм§Җ, 3) 진лӢЁлҗң нӣ„ м№ҳлЈҢ мӢңмһ‘к№Ңм§Җ л“ұмңјлЎң лӮҳлҲҢ мҲҳ мһҲмңјл©°[16] к·ёмӨ‘ лі‘мӣҗ мІҙкі„лЎң м§Җм—°мқ„ мҳҲл°©н• мҲҳ мһҲлҠ” лӢЁкі„к°Җ 3) 진лӢЁлҗң нӣ„ м№ҳлЈҢ мӢңмһ‘мқҙлқјкі ліј мҲҳ мһҲлӢӨ.
л”°лқјм„ң лі‘мӣҗ лӮҙм—җм„ң мӢңн–үн•ң к°қлӢҙ кІ°н•өк· кІҖмӮ¬м—җм„ң к· м–‘м„ұ(лҸ„л§җ/л°°м–‘/PCR)мқҙ нҷ•мқёлҗҳлҠ” кІҪмҡ° к·ё кІ°кіјлҠ” мҰүмӢң лӢҙлӢ№мқҳмӮ¬м—җкІҢ ліҙкі лҗҳм–ҙм•ј н•ңлӢӨ. л№ лҘё ліҙкі лҘј мң„н•ң м—¬лҹ¬ к°Җм§Җ л°©лІ•мқҙ мӢңлҸ„лҗҳм–ҙ мҷ”мңјл©°, к·ё лҢҖн‘ңм Ғмқё л°©лІ•мқҙ мң„н—ҳмҲҳмӨҖ кІҖмӮ¬ кІ°кіјліҙкі (critical value report, CVR)мқҙлӢӨ. кіјкұ°м—җлҠ” м „нҷ”лЎң лі‘лҸҷм—җ м—°лқҪн•ҳлҚҳ л°©мӢқм—җм„ң мөңк·јм—җлҠ” мӣҗлӮҙ м „мӮ°мқҙлӮҳ к°ңмқё н•ёл“ңнҸ° л©”мӢңм§Җ л“ұмқ„ мқҙмҡ©н•ҳм—¬ мӢӨмӢңк°„мңјлЎң лӢҙлӢ№мқҳмӮ¬м—җкІҢ м§Ғм ‘ ліҙкі н•ҳлҠ” л°©лІ•мңјлЎң ліҖнҷ”н•ҳкі мһҲлӢӨ. мөңк·ј көӯлӮҙм—җм„ң л°ңн‘ңлҗң м—°кө¬м—җ мқҳн•ҳл©ҙ м„Өл¬ём—җ мқ‘лӢөн•ң мҙқ 64к°ң мқҳлЈҢкё°кҙҖ мӨ‘ 12к°ңмқҳ мқҳлЈҢкё°кҙҖм—җм„ң н•ӯмӮ°к· лҸ„л§җ кІҖмӮ¬ м–‘м„ұ кІ°кіјлҘј CVRм—җ нҸ¬н•ЁмӢңмјң мӢ мҶҚн•ҳкІҢ ліҙкі н•ҳлҠ” кІғмңјлЎң нҷ•мқёлҗҳм—ҲлӢӨ. м•„м§Ғк№Ңм§Җ CVRм—җ кҙҖн•ң көӯлӮҙм§Җм№ЁмқҖ м—ҶлҠ” мғҒнҷ©мқҙлӮҳ м Җмһҗл“ӨмқҖ лі‘мӣҗм—җм„ң кјӯ н•„мҡ”н•ң CVRм—җ н•ӯмӮ°к· лҸ„л§җ кІҖмӮ¬ м–‘м„ұ н•ӯлӘ©мқҙ л°ҳл“ңмӢң нҸ¬н•Ёлҗҳм–ҙм•ј н•ңлӢӨкі м ңм•Ҳн•ҳмҳҖлӢӨ[17]. н–Ҙнӣ„ лӘЁл“ мқҳлЈҢкё°кҙҖм—җм„ң н•ӯмӮ°к· лҸ„л§җ кІҖмӮ¬ лҝҗл§Ң м•„лӢҲлқј н•ӯмӮ°к· л°°м–‘ кІҖмӮ¬ л°Ҹ MTB PCR кІҖмӮ¬ н•ӯлӘ© м—ӯмӢң CVRм—җ нҸ¬н•ЁмӢңнӮӨлҠ” кІғмқҙ н•„мҡ”н• кІғмңјлЎң мғқк°ҒлҗңлӢӨ.
нҡЁкіјм Ғмқё нҷ”н•ҷмҡ”лІ•мқҙ к°ңл°ңлҗҳкё° м „, кІ°н•ө м№ҳлЈҢлҠ” мӢ м„ н•ң кіөкё°мҷҖ н–Үл№ӣмқ„ мҗ¬л©° мҡ”м–‘мӣҗм—җм„ң ліҙмЎҙм ҒмңјлЎң м№ҳлЈҢн•ҳлҠ” кІғмқҙ м „л¶ҖмҳҖлӢӨ. нҷ”н•ҷмҡ”лІ•мңјлЎңм„ңмқҳ кІ°н•ө м№ҳлЈҢлҠ” 1944л…„ streptomycin (SM)кіј para-aminosalicylic acid (PAS)к°Җ л°ңкІ¬лҗҳл©ҙм„ң 비лЎңмҶҢ мӢңмһ‘лҗҳм—ҲлӢӨ. British Medical Research CouncilмқҖ нҷңлҸҷм„ұ нҸҗкІ°н•ө нҷҳмһҗм—җкІҢ PAS лҳҗлҠ” SM лӢЁлҸ…м№ҳлЈҢ л°Ҹ PAS + SM лі‘н•© м№ҳлЈҢлҘј 비көҗн•ң мөңмҙҲмқҳ л¬ҙмһ‘мң„ лҢҖмЎ° м—°кө¬лҘј мҲҳн–үн•ҳмҳҖмңјл©° кІ°кіј PAS + SM лі‘н•© м№ҳлЈҢк°Җ м№ҳлЈҢ м„ұкіө л°Ҹ м•Ҫм ңлӮҙм„ұ л°ңмғқ мҳҲл°©м—җ лҚ” нҡЁкіјм Ғмқҙлқјкі ліҙкі н•ҳмҳҖлӢӨ[18]. мқҙнӣ„ 1952л…„л¶Җн„° 1960л…„лҢҖ мӨ‘л°ҳм—җ INHк°Җ 추к°Җ к°ңл°ңлҗҳл©ҙм„ң 3м ң м№ҳлЈҢ к°ңл…җмқҙ нҳ•м„ұлҗҳм—Ҳкі , 24к°ңмӣ”к°„мқҳ INH/SM/PAS лі‘н•© м№ҳлЈҢлЎң 90-95%мқҳ мҷ„м№ҳмңЁмқ„ ліҙмҳҖлӢӨ[19]. 1960л…„лҢҖ EMBк°Җ PASлҘј лҢҖмІҙн•ҳл©ҙм„ң м•Ҫм ңмҲңмқ‘лҸ„к°Җ лҶ’м•„мЎҢмңјл©° м№ҳлЈҢ кё°к°„мқ„ 24к°ңмӣ”м—җм„ң 18к°ңмӣ”лЎң лӢЁм¶•мӢңмј°лӢӨ[20]. 1970л…„лҢҖ RIFк°Җ 추к°Җлҗҳл©ҙм„ң INHмқҳ мӮҙк· нҡЁкіјм—җ лҚ”н•ҳм—¬, к°•л Ҙн•ң л©ёк· нҡЁкіјлҘј ліҙмқҙл©° м№ҳлЈҢ кё°к°„мқҙ 18к°ңмӣ”м—җм„ң 9к°ңмӣ”лЎң лӢЁм¶•лҗҳм—Ҳкі [21,22], мқҙнӣ„ мҙҲкё° 2к°ңмӣ”к°„ PZAлҘј мӮ¬мҡ©н•ҳмҳҖмқ„ л•Ң кіөлҸҷлӮҙмқҳ мӮ°м„ұнҷҳкІҪм—җм„ң нҷңл°ңн•ң л©ёк· нҡЁкіјлҘј ліҙмқҙл©° 4м ң 6к°ңмӣ” м№ҳлЈҢмқҳ м„ұм Ғмқҙ мһ…мҰқлҗҳм—ҲлӢӨ[22-24]. к·ё кІ°кіјлЎң INH/RIF/EMB/PZA 6к°ңмӣ” 4м ңмҡ”лІ• нҳ№мқҖ мқҙмқҳ ліҖнҳ•мқҙ м „ м„ёкі„мқҳ мҙҲм№ҳлЈҢ н‘ңмӨҖмІҳл°©мңјлЎң нҷ•лҰҪлҗҳм—Ҳкі , мқҙнӣ„ мғҲлЎңмҡҙ н•ӯкІ°н•өм•Ҫм ңмқҳ к°ңл°ңм—җлҸ„ л¶Ҳкө¬н•ҳкі мқҙ мҙҲм№ҳлЈҢмҡ”лІ•мқҖ 1980л…„лҢҖ мқҙлһҳлЎң 35л…„ мқҙмғҒ ліҖнҷ”к°Җ м—Ҷм—ҲлӢӨ(Fig. 1) [25]. мқҙ нҸҗкІ°н•өм—җ лҢҖн•ң 6к°ңмӣ” н‘ңмӨҖ м№ҳлЈҢмқҳ мҷ„м№ҳмңЁмқҖ 90-95%лЎң лҶ’мңјл©°, м№ҳлЈҢ 24к°ңмӣ” нӣ„ мһ¬л°ңлҘ мқҖ 1-2%лЎң м•Ңл Өм ё мһҲлӢӨ.
к°җмҲҳм„ұ кІ°н•өмқҳ м№ҳлЈҢм—җ лҢҖн•ҙ м„ёкі„м ҒмңјлЎң нҶөмҡ©лҗҳлҠ” 진лЈҢм§Җм№ЁмқҖ WHOм—җм„ң м§ҖлӮң 2017л…„м—җ м ңм•Ҳн•ң м§Җм№Ёмқҙ ліҙнҺём ҒмқҙлӢӨ[26]. мөңмҶҢ 6к°ңмӣ”мқҙ кұёлҰ¬лҠ” 4м ң н‘ңмӨҖ м№ҳлЈҢмқҳ кё°к°„мқҖ м•„м§Ғк№Ңм§Җ лӢЁм¶•лҗҳм§Җ лӘ»н•ҳкі мһҲмңјл©° мһҘкё°к°„мқҳ м№ҳлЈҢлЎң мқён•ң м№ҳлЈҢ мҲңмқ‘лҸ„мқҳ к°җмҶҢлҠ” кІ°н•ө м№ҳлЈҢмқҳ к°ҖмһҘ нҒ° мһҘм• л¬јмқҙлӢӨ. л”°лқјм„ң мҲңмқ‘лҸ„лҘј лҶ’мқҙкё° мң„н•ҙ м§Ғм ‘ кҙҖм°°н•ҳ ліөм•Ҫ м№ҳлЈҢ(directly observed therapy, DOT) к°ңл…җмқҙ м„ёкі„м ҒмңјлЎң л„җлҰ¬ мҠ№мқёлҗҳм—Ҳмңјл©°, мқҙлҘј мҡ©мқҙн•ҳкІҢ н•ҳкё° мң„н•ҙ 4м ң кі м •мҡ©лҹү ліөн•©м ң(fixed dose combination, FDC)мқҳ мӮ¬мҡ© л°Ҹ мЈј 2-3нҡҢмқҳ к°„н—җ м№ҳлЈҢмҡ”лІ•мқҙ мӢңлҸ„лҗҳм—Ҳкі , мқҙлҘј л°”нғ•мңјлЎң к°җмҲҳм„ұ кІ°н•ө н‘ңмӨҖ м№ҳлЈҢм§Җм№Ёмқҙ л§Ңл“Өм–ҙмЎҢлӢӨ(Table 1) [26].
WHO 진лЈҢм§Җм№Ём—җ л”°лҘё к°җмҲҳм„ұ кІ°н•ө мҙҲм№ҳлЈҢмқҳ н‘ңмӨҖмІҳл°©мқҖ 2к°ңмӣ”мқҳ мҙҲкё° 집мӨ‘ м№ҳлЈҢкё°(initial intensive phase)мҷҖ 4к°ңмӣ”мқҳ нӣ„кё° мң м§Җ м№ҳлЈҢкё°(maintenance phase)лЎң к¶Ңкі н•ңлӢӨ(2HREZ/4HR). 2010л…„ 진лЈҢм§Җм№Ём—җм„ң 2017л…„м—җ мғҲлЎӯкІҢ ліҖкІҪлҗң лӮҙмҡ©мқҖ мЈј 3нҡҢмқҳ к°„н—җ м№ҳлЈҢ мҡ©лҹүмқҖ мң м§Җ м№ҳлЈҢкё° лҝҗл§Ң м•„лӢҲлқј 집мӨ‘ м№ҳлЈҢкё° лӘЁл‘җм—җм„ң к¶Ңкі лҗҳм§Җ м•Ҡмңјл©°, кё°мЎҙмқҳ к°ңлі„м•Ҫм ң мІҳл°©ліҙлӢӨ ліөмҡ©н•ҙм•ј н• м•Ҫмқҳ к°ңмҲҳлҘј мӨ„мқё FDCмқҳ мӮ¬мҡ©мқ„ лҚ” к¶Ңкі н•ҳмҳҖлӢӨ. лҳҗн•ң, FQлҘј нҸ¬н•Ён•ң 4к°ңмӣ” лӢЁкё°мҡ”лІ•мқҖ м ҲлҢҖ мӮ¬мҡ©н•ҙм„ңлҠ” м•Ҳ лҗңлӢӨкі к¶Ңкі н•ҳмҳҖлӢӨ. к·ё л°°кІҪмңјлЎңлҠ” 2013л…„, 2014л…„м—җ ліҙкі лҗң 4к°ңмқҳ FQлҘј нҸ¬н•Ён•ң 4к°ңмӣ” лӢЁкё°мҡ”лІ•м—җ лҢҖн•ң л¬ҙмһ‘мң„ лҢҖмЎ° м—°кө¬м—җм„ң лӘЁл‘җ 4м ң 6к°ңмӣ” н‘ңмӨҖ м№ҳлЈҢм—җ лҢҖн•ң 비м—ҙл“ұм„ұмқ„ мһ…мҰқн•ҳм§Җ лӘ»н•ҳмҳҖкі , лҚ” лҶ’мқҖ мһ¬л°ңлҘ мқ„ ліҙмҳҖмңјл©° л¶Җмһ‘мҡ©мқҳ л№ҲлҸ„лҘј мӨ„мқҙм§Җ лӘ»н•ҳмҳҖкё° л•Ңл¬ёмқҙлӢӨ[27-30]. к·ёлҹ¬лӮҳ, 2021л…„ 6мӣ” WHOм—җм„ң л°ңн‘ңн•ң мӢ мҶҚ көҗмӢ (rapid communication)м—җм„ңлҠ” rifapentine (RPT)кіј moxifloxacin (MFX)мқ„ мӮ¬мҡ©н•ң 4к°ңмӣ” лӢЁкё°мҡ”лІ•мқҙ кё°мЎҙ 4м ң 6к°ңмӣ”мҡ”лІ•м—җ м—ҙл“ұн•ҳм§Җ м•Ҡм•„ кё°мЎҙ м№ҳлЈҢмҡ”лІ•мқҳ лҢҖм•Ҳмқҙ лҗ мҲҳ мһҲлӢӨкі м–ёкёүн•ҳм—¬[31], лЁём§Җм•Ҡм•„ м—…лҚ°мқҙнҠёлҗң WHO 진лЈҢм§Җм№Ёмқҙ лӮҳмҳ¬кІғмңјлЎң кё°лҢҖлҗңлӢӨ.
көӯлӮҙ кІ°н•ө진лЈҢм§Җм№Ём—җм„ңлҸ„ к°„н—җ м№ҳлЈҢмҡ”лІ•мқҖ к¶ҢмһҘн•ҳм§Җ м•Ҡмңјл©° к°җмҲҳм„ұ кІ°н•ө мҙҲм№ҳлЈҢмқҳ н‘ңмӨҖмІҳл°©мқҖ 2HREZ/4HR(E)мқҙкі , м•Ҫм ң к°җмҲҳм„ұ кІ°кіј INH л°Ҹ RIFм—җ к°җмҲҳм„ұ кІ°н•өмңјлЎң нҷ•мқёлҗң кІҪмҡ°м—җлҠ” м№ҳлЈҢ 2к°ңмӣ” нӣ„л¶Җн„° EMBмқҳ мӨ‘лӢЁмқ„ кі л Өн•ҳлқјкі к¶Ңкі н•ңлӢӨ[10]. мөңк·ј көӯлӮҙ м—°кө¬м—җм„ң 1) мҙҲкё° 집мӨ‘ м№ҳлЈҢкё°лҘј л§Ҳм№ҳкё° м „ GenoType MTBDRplus assay кІ°кіјм—җ л”°лқј мЎ°кё°м—җ EMBлҘј мӨ‘лӢЁн•ң кө°кіј 2) мҙҲкё° 집мӨ‘ м№ҳлЈҢкё° лҸҷм•Ҳ EMBлҘј мң м§Җн•ң кө°мқҳ м№ҳлЈҢ м„ұкіөлҘ кіј мһ¬л°ңлҘ мқ„ 비көҗн•ң кІ°кіј GenoType MTBDRplus assay кІ°кіјм—җ л”°лҘё EMBмқҳ мЎ°кё° мӨ‘лӢЁмқҙ м№ҳлЈҢмқҳ кІ°кіјм—җ мҳҒн–Ҙмқ„ лҜём№ҳм§Җ м•Ҡм•ҳлӢӨ[32]. л”°лқјм„ң EMBмқҳ м№ҳлЈҢ кё°к°„мқ„ 2к°ңмӣ” лҜёл§ҢмңјлЎң лӢЁм¶•н• мҲҳ мһҲмқ„ к°ҖлҠҘм„ұмқ„ м ңмӢңн•ҳмҳҖлӢӨ. лҳҗн•ң, м№ҳлЈҢ мӢңмһ‘ мӢң нқүл¶Җ Xм„ м—җм„ң кіөлҸҷмқҙ мһҲкі , м№ҳлЈҢ 2к°ңмӣ” мқҙнӣ„ мӢңн–үн•ң к°қлӢҙ л°°м–‘мқҙ м–‘м„ұмқё кІҪмҡ°м—җлҠ” мң м§Җ м№ҳлЈҢ кё°к°„мқҳ м—°мһҘмқ„ кі л Өн• мҲҳ мһҲлӢӨкі к¶Ңкі н•ңлӢӨ[10]. к·ё к·јкұ°лЎң 2014л…„ л°ңн‘ңлҗң көӯлӮҙ лӢЁмқјкё°кҙҖ нӣ„н–Ҙм Ғ м—°кө¬ кІ°кіј 1) нқүл¶Җ Xм„ м—җм„ң кіөлҸҷмқҙ мЎҙмһ¬, 2) 2к°ңмӣ” 집мӨ‘ м№ҳлЈҢ нӣ„ мӢңн–үн•ң к°қлӢҙ л°°м–‘ м–‘м„ұмқҙ лҸҷмӢңм—җ мЎҙмһ¬н•ҳлҠ” кІҪмҡ°к°Җ м№ҳлЈҢ мў…лЈҢ 1л…„ мқҙлӮҙ мһ¬л°ң мң„н—ҳмқёмһҗлЎң нҷ•мқёлҗҳм—Ҳкё° л•Ңл¬ёмқҙлӮҳ, мқҙ м—°кө¬лҠ” мһ¬л°ң нҷҳмһҗмҲҳк°Җ л§Һм§Җ м•Ҡкі (6лӘ…), 3к°ңмӣ” м—°мһҘ м№ҳлЈҢм—җ лҢҖн•ң кІ°кіјк°Җ м—Ҷкё° л•Ңл¬ём—җ мң м§Җ м№ҳлЈҢ кё°к°„мқҳ м—°мһҘмқ„ кі л Өн• мҲҳ мһҲлҗҳ, к°ңлі„ нҷҳмһҗмқҳ мғҒнҷ©мқ„ к°җм•Ҳн•ҳм—¬ нҢҗлӢЁн•ҳлҸ„лЎқ м–ёкёүн•ҳмҳҖлӢӨ[33].
м№ҳлЈҢ кё°к°„мқҳ лӢЁм¶•мқҖ кІ°н•ө кҙҖлҰ¬м—җ мһҲм–ҙ м№ҳлЈҢ мҲңмқ‘лҸ„лҘј лҶ’мқҙкі л¶Җмһ‘мҡ© л°ңмғқлҘ мқ„ лӮ®м¶”кі м№ҳлЈҢ 비мҡ©мқ„ м Ҳк°җн• мҲҳ мһҲлҠ” л§Өмҡ° мӨ‘мҡ”н•ң лӘ©н‘ңмқҙлӢӨ. 2009л…„м—җ кіөлҸҷмқҙ м—Ҷкі м№ҳлЈҢ 2к°ңмӣ” нӣ„ мӢңн–үн•ң к°қлӢҙ л°°м–‘ мқҢм„ұмқё нҸҗкІ°н•ө нҷҳмһҗлҘј лҢҖмғҒмңјлЎң HREZлЎң 2к°ңмӣ” 집мӨ‘ м№ҳлЈҢ нӣ„ HRлЎң мң м§Җ м№ҳлЈҢкё°лҘј 2к°ңмӣ”лЎң лӢЁм¶•н•ҳм—¬ мҙқ 4к°ңмӣ”к°„ м№ҳлЈҢ мў…лЈҢн•ң кө°мқҳ 24к°ңмӣ” нӣ„ мһ¬л°ңлҘ мқ„ 6к°ңмӣ” н‘ңмӨҖ м№ҳлЈҢн•ң кө°кіј 비көҗн•ң кІ°кіј, 4к°ңмӣ” лӢЁм¶• м№ҳлЈҢкө°м—җм„ң лҶ’мқҖ мһ¬л°ңлҘ мқҙ кҙҖм°°лҗҳм–ҙ(7.0% vs. 1.6%; risk difference 0.054; 95% confidence interval with Hauck-Anderson correction 0.01-0.10) м—°кө¬к°Җ мЎ°кё° мў…лЈҢ лҗҳм—ҲлӢӨ[34]. л”°лқјм„ң нҡЁкіјм ҒмңјлЎң м№ҳлЈҢ кё°к°„мқ„ лӢЁм¶•мӢңнӮӨкё° мң„н•ҙм„ңлҠ” кё°мЎҙ м№ҳлЈҢмҷҖ м°Ёлі„нҷ”лҗң л°©лІ•мқҙ н•„мҡ”н•ҳмҳҖмңјл©° мқҙлҘј мң„н•ң мөңк·јмқҳ л…ёл ҘмқҖ FQ нҳ№мқҖ мӢ м•Ҫмқ„ мӮ¬мҡ©н•ҳкұ°лӮҳ нҳ„мһ¬ к¶Ңкі мҡ©лҹүліҙлӢӨ кі мҡ©лҹүмқҳ rifamycinмқ„ мӮ¬мҡ©н•ҳлҠ” лҚ° мҙҲм җмқ„ л§һм¶”кі мһҲлӢӨ.
2013-2014л…„м—җ л„Ө к°ңмқҳ лҢҖк·ңлӘЁ мһ„мғҒ м—°кө¬к°Җ FQлҘј нҸ¬н•Ён•ҳм—¬ 6к°ңмӣ”мқҳ м№ҳлЈҢ кё°к°„мқ„ 4к°ңмӣ”лЎң лӢЁм¶•н•ҳкі мһҗ н•ҳмҳҖмңјлӮҳ FQ нҸ¬н•Ёкө°м—җм„ң м№ҳлЈҢ 2к°ңмӣ” нӣ„ к°қлӢҙ л°°м–‘ мқҢм „мңЁмқҙ лҚ” лҶ’м•ҳмқҢм—җлҸ„ л¶Ҳкө¬н•ҳкі мһ¬л°ңлҘ мқҙ лҶ’м•„ лӘЁл“ м—°кө¬м—җм„ң 6к°ңмӣ” н‘ңмӨҖ м№ҳлЈҢм—җ лҢҖн•ң 비м—ҙл“ұм„ұмқ„ мһ…мҰқн•ҳм§Җ лӘ»н•ҳмҳҖмңјл©° л¶Җмһ‘мҡ© л№ҲлҸ„ л°Ҹ мӮ¬л§қлҘ мқҳ м°Ёмқҙ м—ӯмӢң ліҙм—¬мЈјм§Җ лӘ»н•ҳмҳҖлӢӨ[27-30]. 1) 2013л…„ л°ңн‘ңлҗң л¬ҙмһ‘мң„ лҢҖмЎ°кө° м—°кө¬м—җм„ң 집мӨ‘ м№ҳлЈҢкё° л•Ң EMBлҘј MFX 400 mg (2HRMZ/2HRM) нҳ№мқҖ gatifloxacin (GFX) 400 mg (2HRGZ/2HRG)мңјлЎң лҢҖмІҙн•ҳкі мң м§Җ м№ҳлЈҢкё° л•Ң FQлҘј мң м§Җн•ҳм—¬ мЈј 3нҡҢ мҙқ 4к°ңмӣ” м№ҳлЈҢ нӣ„ лҢҖмЎ°кө°(2HREZ/4HR)кіј л°°м–‘мқҢм „мңЁкіј мһ¬л°ңлҘ мқ„ 비көҗн•ң кІ°кіј м№ҳлЈҢ нӣ„ мһ¬л°ңлҘ мқҙ 6к°ңмӣ” н‘ңмӨҖ м№ҳлЈҢмқҳ 6%м—җ 비н•ҳм—¬ GFXкө°мқҙ 16%, MFXкө°мқҙ 10%лЎң лҚ” лҶ’м•„ м—°кө¬к°Җ мЎ°кё° мў…лЈҢ лҗҳм—ҲлӢӨ[27]. 2014л…„м—җ New England Journal of Medicine (NEJM)м—җ 6к°ңмӣ” н‘ңмӨҖ м№ҳлЈҢлҘј лҢҖмЎ°кө°мңјлЎң FQ нҸ¬н•Ё 4к°ңмӣ” лӢЁкё°мҡ”лІ•м—җ лҢҖн•ң 3к°ңмқҳ лҢҖк·ңлӘЁ л¬ҙмһ‘мң„ лҢҖмЎ°кө° м—°кө¬к°Җ л°ңн‘ңлҗҳм—Ҳмңјл©°, лЁјм Җ 2) REMoxTB м—°кө¬м—җм„ңлҠ” лҢҖмЎ°кө° н‘ңмӨҖмІҳл°©м—җм„ң EMBк°Җ MFXлЎң лҢҖмІҙлҗң INHкө°кіј INHк°Җ MFXлЎң лҢҖмІҙлҗң EMBкө°мңјлЎң лӮҳлҲ„м–ҙ мҙқ 17мЈјк°„ м№ҳлЈҢн•ҳм—¬ лҢҖмЎ°кө°(2HREZ/4HR), INHкө°(2HRMZ/2HRM), EMBкө°(2MREZ/2MR)мқҳ м„ё кө°мқ„ 비көҗн•ҳмҳҖлӢӨ. м№ҳлЈҢ мў…лЈҢ 12к°ңмӣ” нӣ„ м№ҳлЈҢ мӢӨнҢЁ л°Ҹ мһ¬л°ңмқ„ нҸ¬н•Ён•ң 1м°Ё нҸүк°ҖліҖмҲҳлҠ” 4к°ңмӣ” лӢЁм¶• м№ҳлЈҢк°Җ 6к°ңмӣ” н‘ңмӨҖ м№ҳлЈҢм—җ лҢҖн•ң 비м—ҙл“ұм„ұмқ„ мһ…мҰқн•ҳм§Җ лӘ»н•ҳмҳҖмңјлӮҳ к°қлӢҙ м•ЎмІҙ л°Ҹ кі мІҙл°°м§Җ л°°м–‘м—җм„ң лӘЁл‘җ лҚ” л№ лҘё мқҢм „мқ„ ліҙм—¬мЈјм—ҲлӢӨ. м„ё к·ёлЈ№м—җм„ң 3, 4лӢЁкі„ л¶Җмһ‘мҡ© л°ңмғқлҘ мқҳ мң мқҳн•ң м°ЁмқҙлҠ” м—Ҷм—ҲлӢӨ[28]. 3) OFLOTUB м—°кө¬лҠ” лҢҖмЎ°кө° н‘ңмӨҖмІҳл°©(2HREZ/4HR)м—җм„ң EMB лҢҖмӢ GFX 400 mg (2HRGZ/2HRG)мңјлЎң лҢҖмІҙн•ҳкі мҙқ 4к°ңмӣ”к°„ м№ҳлЈҢн•ң л’Ө лҢҖмЎ°кө°кіј 비көҗн•ҳмҳҖлӢӨ. м№ҳлЈҢ мў…лЈҢ 24к°ңмӣ” нӣ„ 1м°Ё нҸүк°ҖліҖмҲҳлҠ” м№ҳлЈҢ мӢӨнҢЁ, мһ¬л°ң, мӮ¬л§қ, мӨ‘лҸ„нғҲлқҪмқ„ нҸ¬н•Ён•ҳмҳҖмңјл©° ліё м—°кө¬ м—ӯмӢң лҢҖмЎ°кө°м—җ 비н•ҙ 비м—ҙл“ұм„ұмқ„ мһ…мҰқн•ҳм§Җ лӘ»н•ҳмҳҖлӢӨ[29]. 4) RIFAQUIN м—°кө¬лҠ” лҢҖмЎ°кө° н‘ңмӨҖмІҳл°©(2HREZ/4HR)м—җм„ң INH лҢҖмӢ MFXлЎң лҢҖмІҙн•ҳм—¬ 2к°ңмӣ” 집мӨ‘ м№ҳлЈҢк°Җ лҒқлӮң л’Ө 4к°ңмӣ”кө°м—җм„ңлҠ” мң м§Җ м№ҳлЈҢкё°лҘј RPT 900 mgкіј MFXлҘј мЈј 2нҡҢлЎң 2лӢ¬к°„ мң м§Җн•ҳм—¬ мҙқ 4к°ңмӣ”к°„ м№ҳлЈҢн•ҳмҳҖкі 6к°ңмӣ”кө°м—җм„ңлҠ” мң м§Җ м№ҳлЈҢкё°лҘј RPT 1,200 mgкіј MFXлҘј мЈј 1нҡҢлЎң 4к°ңмӣ”к°„ мң м§Җн•ҳм—¬ мҙқ 6к°ңмӣ”к°„ м№ҳлЈҢн•ҳм—¬ лҢҖмЎ°кө°(2HREZ/4HR), 4к°ңмӣ”кө°(2MREZ/2MPtwice weekly), 6к°ңмӣ”кө°(2MREZ/4MPonce weekly)мқҳ м„ё кө°мқ„ 비көҗн•ҳмҳҖлӢӨ. м№ҳлЈҢ мў…лЈҢ 12к°ңмӣ” нӣ„ 1м°Ё нҸүк°ҖліҖмҲҳлҠ” м№ҳлЈҢ мӢӨнҢЁ, мһ¬л°ң, мӮ¬л§қ, мӨ‘лҸ„нғҲлқҪмқ„ нҸ¬н•Ён•ҳмҳҖмңјл©°, ліё м—°кө¬ м—ӯмӢң 4к°ңмӣ”кө°мқҖ лҢҖмЎ°кө°м—җ 비н•ҙ 비м—ҙл“ұм„ұмқ„ мһ…мҰқн•ҳм§Җ лӘ»н•ҳмҳҖлӢӨ[30]. н•ҳм§Җл§Ң кіөлҸҷмқҙ м—ҶлҠ” нҸҗкІ°н•ө нҷҳмһҗл§Ң н•ҳмң„ к·ёлЈ№ 분м„қмқ„ 진н–үн•ҳмҳҖмқ„ л•Ңмқҳ м№ҳлЈҢ нҡЁкіјлҠ” 6к°ңмӣ” н‘ңмӨҖ м№ҳлЈҢм—җ л’Өм§Җм§Җ м•Ҡм•ҳлӢӨ[35]. лҳҗ лӢӨлҘё м—°кө¬м—җм„ң мң„мқҳ м—°кө¬лҘј л°”нғ•мңјлЎң patient-level pooled analysisлҘј мӢңн–үн•ң кІ°кіј к°қлӢҙ н•ӯмӮ°к· лҸ„л§җ < 2 + нҳ№мқҖ кіөлҸҷмқҙ м—ҶлҠ” лҜём„ёлі‘ліҖ(minimal disease)мқ„ к°Җ진 нҷҳмһҗм—җм„ңлҠ” 4к°ңмӣ” м№ҳлЈҢк°Җ 6к°ңмӣ” н‘ңмӨҖ м№ҳлЈҢм—җ л’Өм§Җм§Җ м•ҠлҠ” кІғмқ„ ліҙм—¬мЈјм—ҲлӢӨ[36]. м•һм„ л„Ө к°Җм§Җ мһ„мғҒмӢңн—ҳмқ„ нҶөн•ҙ FQлҘј нҸ¬н•Ён•ң 4к°ңмӣ” лӢЁкё°мҡ”лІ•мқҖ 2017л…„ WHO 진лЈҢм§Җм№Ём—җм„ң мӮ¬мҡ©н•ҙм„ңлҠ” м•Ҳ лҗҳлҠ” м№ҳлЈҢлІ•мңјлЎң к¶Ңкі лҗҳм—ҲлӢӨ. н•ҳм§Җл§Ң мөңк·ј 2021л…„ 5мӣ” NEJMм—җ мғҲлЎңмҡҙ л¬ҙмһ‘мң„ лҢҖмЎ°кө° м—°кө¬к°Җ л°ңн‘ңлҗҳм–ҙ WHO 진лЈҢм§Җм№Ёмқҳ ліҖнҷ”лҘј мҳҲкі н•ҳмҳҖлӢӨ. Study 31/A5349 м—°кө¬лҠ” лҢҖмЎ°кө° н‘ңмӨҖмІҳл°©(2HREZ/4HR)м—җм„ң RIFлҘј RPT (1,200 mg, once-daily)лЎң лҢҖмІҙн•ҳм—¬ 2к°ңмӣ” 집мӨ‘ м№ҳлЈҢ нӣ„ 2к°ңмӣ” мң м§Җ м№ҳлЈҢлҘј л§Ҳм№ң RPTкө°(2HPZE/2HP)кіј RIFлҘј RPTлЎң лҢҖмІҙн•ҳкі EMBлҘј MFX 400 mgмңјлЎң лҢҖмІҙн•ҳм—¬ 2к°ңмӣ” 집мӨ‘ м№ҳлЈҢмҷҖ 2к°ңмӣ” мң м§Җ м№ҳлЈҢк№Ңм§Җ MFXлҘј мң м§Җн•ң RPT/MFXкө°(2HPZM/2HPM)мңјлЎң лӮҳлҲ„м–ҙ м„ё кө°мқ„ 비көҗн•ҳмҳҖлӢӨ. м№ҳлЈҢ мў…лЈҢ 6к°ңмӣ” нӣ„ 1м°Ё нҸүк°ҖліҖмҲҳлҠ” м№ҳлЈҢ мӢӨнҢЁ, мһ¬л°ң, мӮ¬л§қ, мӨ‘лҸ„нғҲлқҪмқ„ нҸ¬н•Ён•ҳмҳҖмңјл©° RPTкө°м—җм„ңлҠ” 비м—ҙл“ұм„ұмқ„ мһ…мҰқн•ҳм§Җ лӘ»н•ҳмҳҖмңјлӮҳ RPT/MFXкө°м—җм„ңлҠ” 비м—ҙл“ұм„ұмқҙ мһ…мҰқлҗҳм—ҲлӢӨ[37]. л”°лқјм„ң мқҙ м—°кө¬мқҳ кІ°кіјлҘј л°”нғ•мңјлЎң 2021л…„ 6мӣ” WHOм—җм„ң RPT/MFX 4к°ңмӣ” лӢЁкё°мҡ”лІ•м—җ лҢҖн•ң rapid communicationмқ„ л°ңн‘ңн•ҳмҳҖмңјл©° к·јмӢңмқј лӮҙм—җ RPT/MFX 4к°ңмӣ” лӢЁкё°мҡ”лІ•мқҙ кё°мЎҙ н‘ңмӨҖ м№ҳлЈҢмқҳ лҢҖм•Ҳмқҙ лҗ мҲҳ мһҲлӢӨлҠ” WHO 진лЈҢм§Җм№Ёмқҳ к°ңм •мқҙ нҳ„мһ¬ мҳҲкі лҗң мғҒнғңмқҙлӢӨ(Table 2).
RPTлҠ” rifamycin кі„нҶөмқҳ м•Ҫл¬јлЎң RIFмҷҖ н•ӯкІ°н•өнҡЁкіјлҠ” мң мӮ¬н•ҳлӢӨкі м•Ңл Өм ё мһҲмңјлӮҳ л°ҳк°җкё°к°Җ кёём–ҙ мЈј 1нҡҢ ліөмҡ©мқҙ к°ҖлҠҘн•ң м•Ҫм ңмқҙлӢӨ. мқҙлҹ¬н•ң мһҘм җмңјлЎң RPTлҠ” мһ ліөкІ°н•ө м№ҳлЈҢм—җм„ң нҡҚкё°м ҒмңјлЎң мӮ¬мҡ©лҗҳкі мһҲлҠ” м•Ҫм ңлЎңм„ң 2011л…„ л§җм—җ WHOм—җм„ңлҠ” INH/RPT 3к°ңмӣ” к°„н—җмҡ”лІ•(мЈј 1нҡҢ, мҙқ 12мЈј)мқ„ мһ ліөкІ°н•өк°җм—ј м№ҳлЈҢмқҳ мғҲлЎңмҡҙ мҡ”лІ•мңјлЎң к¶Ңкі н•ҳмҳҖкі нҳ„мһ¬ н•ҙмҷём—җм„ңлҠ” нҷңл°ңнһҲ мӮ¬мҡ© мӨ‘мқҙлӢӨ. көӯлӮҙм—җлҠ” 2016л…„ л§җм—җ кёҙкёү лҸ„мһ…лҗҳм–ҙ м ңн•ңлҗң лі‘В·мқҳмӣҗ мқҳлЈҢ진мқҳ мһ ліөкІ°н•өк°җм—ј м№ҳлЈҢм—җ мӮ¬мҡ©лҗҳм—ҲмңјлӮҳ, м—°кө¬ кІ°кіј м•Ҫл¬ј ліөмҡ©мҷ„лЈҢмңЁмқҖ INH/RPT к°„н—җмҡ”лІ•мқҙ 3к°ңмӣ” INH/RIF лі‘н•©мҡ”лІ•(л§Өмқј)м—җ 비н•ҳм—¬ лҶ’м•ҳмңјлӮҳ(92.9% vs. 86.7%, p=0.036), л¶Җмһ‘мҡ©мқҳ л№ҲлҸ„(нҠ№нһҲ лҸ…к°җм–‘ мҰқнӣ„кө° 19.0%, м•„лӮҳн•„лқҪмӢңмҠӨ 1.8%)к°Җ INH/RPT к°„н—җмҡ”лІ•м—җм„ң нҳ„м ҖнһҲ лҶ’кІҢ нҷ•мқёлҗҳм–ҙ(75.2% vs. 56.7%, p<0.001) м—°кө¬к°Җ мӨ‘лӢЁлҗҳм—ҲлӢӨ. л”°лқјм„ң нҳ„мһ¬ көӯлӮҙм—җм„ңлҠ” RPTмқҳ мӮ¬мҡ©мқҖ л¶Ҳк°ҖлҠҘн•ң мғҒнғңмқҙлӢӨ[38].
мң„мҷҖ к°ҷмқҙ FQлҘј мӮ¬мҡ©н•ң м—°кө¬ лҝҗл§Ң м•„лӢҲлқј нҳ„мһ¬ к¶Ңкі мҡ©лҹүліҙлӢӨ кі мҡ©лҹүмқҳ rifamycinмқ„ мӮ¬мҡ©н•ҳкұ°лӮҳ мӢ м•Ҫмқ„ мӮ¬мҡ©н•ҳм—¬ м№ҳлЈҢ кё°к°„мқ„ лӢЁм¶•мӢңнӮӨл ӨлҠ” м—°кө¬ м—ӯмӢң 진н–үлҗҳкі мһҲлӢӨ[39]. к·ёмӨ‘ н•ҳлӮҳк°Җ RIFASHORT м—°кө¬лЎң RIF 600 mg 6к°ңмӣ” м№ҳлЈҢ(2HREZ/4HR)мҷҖ RIF 1,200 mg (2HREZ/2HR1,200 mg), RIF 1,800 mg (2HREZ/2HR1,800 mg)мңјлЎң лҢҖмІҙн•ҳм—¬ мҙқ 4к°ңмӣ”к°„ м№ҳлЈҢн•ҳм—¬ м„ёкө°мқ„ 비көҗн•ҳлҠ” м—°кө¬мқҙлӢӨ. лҳҗ лӢӨлҘё м—°кө¬лЎңлҠ” мғҲлЎңмҡҙ м—°кө¬м„Өкі„лІ•мқё multi-arm, multi-stage (MAMS) м„Өкі„л°©мӢқмқ„ лҸ„мһ…н•ң TRUNCATE-TB м—°кө¬к°Җ мһҲлӢӨ. мқҙ м—°кө¬лҠ” м•Ҫм ңмқҳ мҲҳк°Җ м ңн•ңм Ғмқҙкі , кІ°н•ө нҷҳмһҗмқҳ мҲҳк°Җ л§Һм•ҳлҚҳ кіјкұ°м—җ 비н•ҙ нҳ„мһ¬мқҳ н•ңм •лҗң мһҗмӣҗмқ„ мөңм Ғнҷ”н•ҳм—¬ мһ„мғҒ м—°кө¬ м„ұкіө к°ҖлҠҘм„ұмқ„ лҶ’мқҙкё° мң„н•ҙ мғҲлЎңмҡҙ м—°кө¬ л°©лІ•мқ„ мӮ¬мҡ©н•ҳмҳҖлӢӨ. TRUNCATE-TB м—°кө¬лҠ” Arm1лЎң RIF 10 mg/kgмқё н‘ңмӨҖ 6к°ңмӣ” м№ҳлЈҢ(2HREZ/4HR)мҷҖ Arm2лЎң н‘ңмӨҖ мІҳл°©м—җ RIF 35 mg/kgмңјлЎң мҰқлҹү + linezolidлҘј 추к°Җ(2HR35ZELi), Arm3лЎң н‘ңмӨҖмІҳл°©м—җ RIF 35 mg/kgмңјлЎң мҰқлҹү + clofazimineмқ„ 추к°Җ(2HR35ZEC), Arm4лЎң н‘ңмӨҖмІҳл°©м—җ EMB лҢҖмӢ linezolidмҷҖ levofloxacinмқ„ 추к°Җ(2HPZLiLe), Arm5лЎң н‘ңмӨҖмІҳл°©м—җ RIF лҢҖмӢ linezolidмҷҖ bedaquilineмқ„ 추к°Җ(2HZELiB)н•ҳлҠ” 5кө°мңјлЎң м—°кө¬лҘј мӢңмһ‘н•ҳм—¬ 2к°ңмӣ” кё°мӨҖмңјлЎң мӨ‘к°„ 분м„қмқ„ мӢңн–ү, мҙҲкё° мӨ‘к°„ кІ°кіјм—җм„ң 충분н•ң нҡЁкіјлҘј ліҙмқҙм§Җ лӘ»н•ң кө°мқҖ мҲңм°Ём ҒмңјлЎң лӘЁм§‘мқ„ мӨ‘лӢЁн•ҳкі лҢҖмЎ°кө°кіј к°ҖлҠҘм„ұмқҙ ліҙмқҙлҠ” кө°мқҖ лҒқк№Ңм§Җ лӘЁм§‘мқ„ мң м§Җн•ҳлҠ” л°©лІ•мңјлЎң нҳ„мһ¬ м—°кө¬к°Җ 진н–ү мӨ‘мқҙлӢӨ.
мөңк·јм—җлҠ” кіјкұ°м—җ м•Ҫм ңк°җмҲҳм„ұ кІ°н•өмқҖ 1м°Ё м•Ҫм ң, м•Ҫм ңлӮҙм„ұ кІ°н•өмқҖ 2м°Ё м•Ҫм ңлЎң м№ҳлЈҢн•ҳлҚҳ кё°мЎҙ мӣҗм№ҷм—җм„ң лІ—м–ҙлӮҳ к°җмҲҳм„ұ кІ°н•ө л°Ҹ м•Ҫм ңлӮҙм„ұкІ°н•өмқ„ кө¬лі„н•ҳм§Җ м•Ҡкі лӘЁл‘җ м№ҳлЈҢн• мҲҳ мһҲлҠ” PAN-TB regimenм—җ лҢҖн•ң к°ңл°ңлҸ„ нҷңл°ңн•ҳлӢӨ. к·ё мҳҲлЎң Simplici TB м—°кө¬лҠ” Arm1лЎң н‘ңмӨҖ 6к°ңмӣ” м№ҳлЈҢ(2HREZ/4HR)лҘј лҢҖмЎ°кө°мңјлЎң Arm2лҠ” м•Ҫм ңк°җмҲҳм„ұ кІ°н•өмқ„ лҢҖмғҒмңјлЎң 집мӨ‘ м№ҳлЈҢкё°м—җлҠ” bedaquiline 200 mg, protomanid, MFX, PZA (2B200PaMZ)лҘј 2к°ңмӣ”к°„ мӮ¬мҡ©н•ҳкі мң м§Җ м№ҳлЈҢкё°лҠ” к°ҷмқҖ м•Ҫм ңмЎ°н•©мңјлЎң н•ҳлҗҳ bedaquilineмқ„ 100 mgмңјлЎң к°җлҹү(2B100PaMZ)н•ҳм—¬ 2к°ңмӣ”к°„ м№ҳлЈҢн•ҳм—¬ мҙқ 4к°ңмӣ” м№ҳлЈҢкө°мқҙл©°, Arm3лҠ” лӢӨм ңлӮҙм„ұ кІ°н•өмқ„ лҢҖмғҒмңјлЎң 집мӨ‘ м№ҳлЈҢкё°лҠ” Arm2мҷҖ лҸҷмқј(2B200PaMZ)н•ҳкі мң м§Җ м№ҳлЈҢкё°лҘј Arm2мқҳ 2к°ңмӣ”м—җм„ң 4к°ңмӣ”лЎң м—°мһҘ(4B100PaMZ)мӢңмјң мҙқ 6к°ңмӣ” м№ҳлЈҢкө°, мқҙл ҮкІҢ м„ё кө°мңјлЎң лӮҳлҲ„м–ҙ нҳ„мһ¬ м—°кө¬к°Җ 진н–ү мӨ‘мқҙлӢӨ. к·ё л°–м—җ м—¬лҹ¬ м—°кө¬л“Өмқҙ м№ҳлЈҢ кё°к°„ лӢЁм¶•мқ„ мң„н•ҙ 진н–ү мӨ‘м—җ мһҲлӢӨ[39].
л№ лҘҙкІҢ ліҖн•ҳлҠ” лӢӨм ңлӮҙм„ұ кІ°н•өмқҳ м№ҳлЈҢм—җ 비н•ҙ к°җмҲҳм„ұ кІ°н•өмқҳ м№ҳлЈҢлҠ” 35л…„к°„ нҒ° ліҖнҷ”к°Җ м—Ҷмқҙ мң м§Җлҗҳм—Ҳкі , 6к°ңмӣ”мқҙлқјлҠ” кёҙ м№ҳлЈҢ кё°к°„кіј мқҙлЎң мқён•ң л¶Җмһ‘мҡ© мҰқк°Җ л°Ҹ м•Ҫм ңмҲңмқ‘лҸ„ к°җмҶҢ л“ұмқҳ л¬ём ңмҷҖ мқҙлҘј н•ҙкІ°н•ҳкё° мң„н•ң л§ҺмқҖ л…ёл Ҙмқҙ мһҲм–ҙ мҷ”лӢӨ. нҳ„мһ¬ к°җмҲҳм„ұ кІ°н•өмқҳ 진лЈҢм§Җм№ЁмқҖ н”„лЎңк·ёлһЁ мӨ‘мӢ¬м Ғмқҙкі , лӢЁмҲңн•ҳл©° лӘЁл“ нҷҳмһҗм—җ мқјлҘ м Ғмқё вҖҳone-size-fits-allвҖҷ 6к°ңмӣ” н‘ңмӨҖ м№ҳлЈҢлЎңмҚЁ мқјл¶Җмқҳ нҷҳмһҗм—җкІҢлҠ” н•„мҡ”н•ң кІғліҙлӢӨ кёҙ м№ҳлЈҢлқјлҠ” кІғмқҖ 분лӘ…н•ҳлӢӨ. кёҙ м„ёмӣ” лҸҷм•Ҳ м№ҳлЈҢ кё°к°„мқ„ 4к°ңмӣ”лЎң лӢЁм¶•мӢңмјңліҙл ӨлҠ” м—¬лҹ¬ м—°кө¬к°Җ лҢҖл¶Җ분 мӢӨнҢЁлЎң лҸҢм•„к°”мңјлӮҳ мөңк·јм—җ л°ңн‘ңлҗң Study 31/A5349 м—°кө¬ кІ°кіј RPTмҷҖ MFXлҘј мӮ¬мҡ©н•ң 4к°ңмӣ” лӢЁкё° мҡ”лІ•мқҙ кё°мЎҙ 4м ң 6к°ңмӣ”мҡ”лІ•м—җ м—ҙл“ұн•ҳм§Җ м•ҠлӢӨлҠ” кІғмқҙ нҷ•мқёлҗҳм–ҙ к·јмӢңмқј лӮҙм—җ RPT/MFX 4к°ңмӣ” лӢЁкё°мҡ”лІ•мқҙ кё°мЎҙ н‘ңмӨҖ м№ҳлЈҢмқҳ лҢҖм•Ҳмқҙ лҗ мҲҳ мһҲлӢӨлҠ” WHO 진лЈҢм§Җм№Ёмқҳ к°ңм •мқҙ нҳ„мһ¬ мҳҲкі лҗң мғҒнғңмқҙлӢӨ. н•ҳм§Җл§Ң RPTк°Җ лҶ’мқҖ л№ҲлҸ„мқҳ л¶Җмһ‘мҡ©мңјлЎң мқён•ҙ нҳ„мһ¬ көӯлӮҙм—җм„ңлҠ” мӮ¬мҡ©мқҙ м ңн•ңм Ғмқё мғҒнҷ©мңјлЎң көӯлӮҙм—җм„ң RPT/MFX 4к°ңмӣ” лӢЁкё°мҡ”лІ•мқҙ л°”лЎң м Ғмҡ©лҗҳкё°лҠ” м–ҙл Өмҡё кІғмңјлЎң ліҙмқёлӢӨ.
REFERENCES
1. Diagnostic standards and classification of tuberculosis in adults and children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med 2000;161:1376вҖ“1395.


2. Park YS, Lee CH, Lee SM, et al. Rapid increase of non-tuberculous mycobacterial lung diseases at a tertiary referral hospital in South Korea. Int J Tuberc Lung Dis 2010;14:1069вҖ“1071.

3. Kanchana MV, Cheke D, Natyshak I, Connor B, Warner A, Martin T. Evaluation of the BACTEC MGIT 960 system for the recovery of mycobacteria. Diagn Microbiol Infect Dis 2000;37:31вҖ“36.


4. Centers for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep 2009;58:7вҖ“10.


5. MacLean E, Kohli M, Weber SF, et al. Advances in molecular diagnosis of tuberculosis. J Clin Microbiol 2020;58:e01582вҖ“19.



6. Joint Committee for the Revision of Korean Guidelines for Tuberculosis Korea Centers for Disease Control and Prevention. Korean guidelines for tuberculosis. Seoul: The Korean Academy of Tuberculosis and Respiratory Diseases, 2011.
7. Joint Committee for the Revision of Korean Guidelines for Tuberculosis Korea Centers for Disease Control and Prevention. Korean guidelines for tuberculosis. 3rd ed. Seoul: The Korean Academy of Tuberculosis and Respiratory Diseases, 2017.
8. Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 2010;363:1005вҖ“1015.



9. Joint Committee for the Revision of Korean Guidelines for Tuberculosis Korea Centers for Disease Control and Prevention. Korean guidelines for tuberculosis. 2nd ed. Seoul: The Korean Academy of Tuberculosis and Respiratory Diseases, 2014.
10. Joint Committee for the Revision of Korean Guidelines for Tuberculosis Korea Centers for Disease Control and Prevention. Korean guidelines for tuberculosis. 4th ed. Seoul: The Korean Academy of Tuberculosis and Respiratory Diseases, 2020.
11. Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. XpertВ® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2014;2014:CD009593.


12. Zifodya JS, Kreniske JS, Schiller I, et al. Xpert Ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis. Cochrane Database Syst Rev 2021;2:CD009593.


13. Ramaswamy S, Musser JM. Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 update. Tuber Lung Dis 1998;79:3вҖ“29.


14. Bai Y, Wang Y, Shao C, Hao Y, Jin Y. GenoType MTBDRplus assay for rapid detection of multidrug resistance in mycobacterium tuberculosis: a meta-analysis. PLoS One 2016;11:e0150321.



15. Mao X, Ke Z, Shi X, et al. Diagnosis of drug resistance to fluoroquinolones, amikacin, capreomycin, kanamycin and ethambutol with genotype MTBDRsl assay: a meta-analysis. Ann Clin Lab Sci 2015;45:533вҖ“544.

16. Kang SM, Lee JG, Chung JH, et al. Delayed treatment of pulmonary tuberculosis in a university hospital. Tuberc Respir Dis 2006;60:277вҖ“284.

17. Song JK, Ahn HJ, Kim YA. Critical value report: survey and literature review. J Lab Med Qual Assur 2017;39:31вҖ“41.

18. Treatment of pulmonary tuberculosis with streptomycin and para-aminosalicylic acid; a Medical Research Council investigation. Br Med J 1950;2:1073вҖ“1085.



19. Various combinations of isoniazid with streptomycin or with P.A.S. in the treatment of pulmonary tuberculosis; seventh report to the Medical Research Council by their Tuberculosis Chemotherapy Trials Committee. Br Med J 1955;1:435вҖ“445.


20. Doster B, Murray FJ, Newman R, Woolpert SF. Ethambutol in the initial treatment of pulmonary tuberculosis. U.S. Public Health Service tuberculosis therapy trials. Am Rev Respir Dis 1973;107:177вҖ“190.

21. Controlled clinical trial of four 6-month regimens of chemotherapy for pulmonary tuberculosis. Second report. Second East African/British Medical Research Council Study. Am Rev Respir Dis 1976;114:471вҖ“475.

22. Controlled trial of 6-month and 8-month regimens in the treatment of pulmonary tuberculosis: the results up to 24 months. Am Rev Respir Dis 1976;114:471вҖ“475.

23. Controlled trial of 4 three-times-weekly regimens and a daily regimen all given for 6 months for pulmonary tuberculosis. Second report: the results up to 24 months. Hong Kong Chest Service/British Medical Research Council. Tubercle 1982;63:89вҖ“98.


24. Long-term follow-up of a clinical trial of six-month and four-month regimens of chemotherapy in the treatment of pulmonary tuberculosis. Singapore Tuberculosis Service/British Medical Research Council. Am Rev Respir Dis 1986;133:779вҖ“783.

25. Iseman MD. Tuberculosis therapy: past, present and future. Eur Respir J Suppl 2002;36:87sвҖ“94s.


26. World Health Organization. Guidelines for treatment of drug-susceptible tuberculosis and patient care: 2017 update [Internet]. Geneva: WHO, c2017. [cited 2021 Jul 20]. Available from: https://apps.who.int/iris/bitstream/handle/10665/255052/9789241550000-eng.pdf
27. Jawahar MS, Banurekha VV, Paramasivan CN, et al. Randomized clinical trial of thrice-weekly 4-month moxifloxacin or gatifloxacin containing regimens in the treatment of new sputum positive pulmonary tuberculosis patients. PLoS One 2013;8:e67030.



28. Gillespie SH, Crook AM, McHugh TD, et al. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med 2014;371:1577вҖ“1587.



29. Merle CS, Fielding K, Sow OB, et al. A four-month gatifloxacin-containing regimen for treating tuberculosis. N Engl J Med 2014;371:1588вҖ“1598.


30. Jindani A, Harrison TS, Nunn AJ, et al. High-dose rifapentine with moxifloxacin for pulmonary tuberculosis. N Engl J Med 2014;371:1599вҖ“1608.



31. World Health Organization. Treatment of drug-susceptible tuberculosis: rapid communication [Internet]. Geneva: WHO, c2021. [cited 2021 Jul 20]. Available from: https://apps.-who.int/iris/bitstream/handle/10665/341729/9789240028678-eng.pdf?sequence=1
32. Jo KW, Kim M, Kim YJ, et al. Early discontinuation of ethambutol in pulmonary tuberculosis treatment based on results of the GenoType MTBDRplus assay: a prospective, multicenter, non-inferiority randomized trial in South Korea. Antimicrob Agents Chemother 2019;63:e00980вҖ“19.


33. Jo KW, Yoo JW, Hong Y, et al. Risk factors for 1-year relapse of pulmonary tuberculosis treated with a 6-month daily regimen. Respir Med 2014;108:654вҖ“659.


34. Johnson JL, Hadad DJ, Dietze R, et al. Shortening treatment in adults with noncavitary tuberculosis and 2-month culture conversion. Am J Respir Crit Care Med 2009;180:558вҖ“563.



35. Alipanah N, Cattamanchi A, Menzies R, Hopewell PC, Chaisson RE, Nahid P. Treatment of non-cavitary pulmonary tuberculosis with shortened fluoroquinolone-based regimens: a meta-analysis. Int J Tuberc Lung Dis 2016;20:1522вҖ“1528.


36. Imperial MZ, Nahid P, Phillips PPJ, et al. A patient-level pooled analysis of treatment-shortening regimens for drug-susceptible pulmonary tuberculosis. Nat Med 2018;24:1708вҖ“1715.



37. Dorman SE, Nahid P, Kurbatova EV, et al. Four-month rifapentine regimens with or without moxifloxacin for tuberculosis. N Engl J Med 2021;384:1705вҖ“1718.


38. Jo KW, Kim JS, Kwon HS, et al. Adverse event and treatment completion rates of a 12-dose weekly isoniazid and rifapentine course for South Korean healthcare workers. Respir Med 2019;158:42вҖ“48.


Landmarks in pulmonary tuberculosis therapy. INH, isoniazid; PAS, para-aminosalicylic acid; SM, streptomycin; EMB, ethambutol; RIF, rifampicin; PZA, pyrazinamide; H, isoniazid; R, rifampicin; Z, pyrazinamide; E, ethambutol; P, rifapentine; M, moxifloxacin.
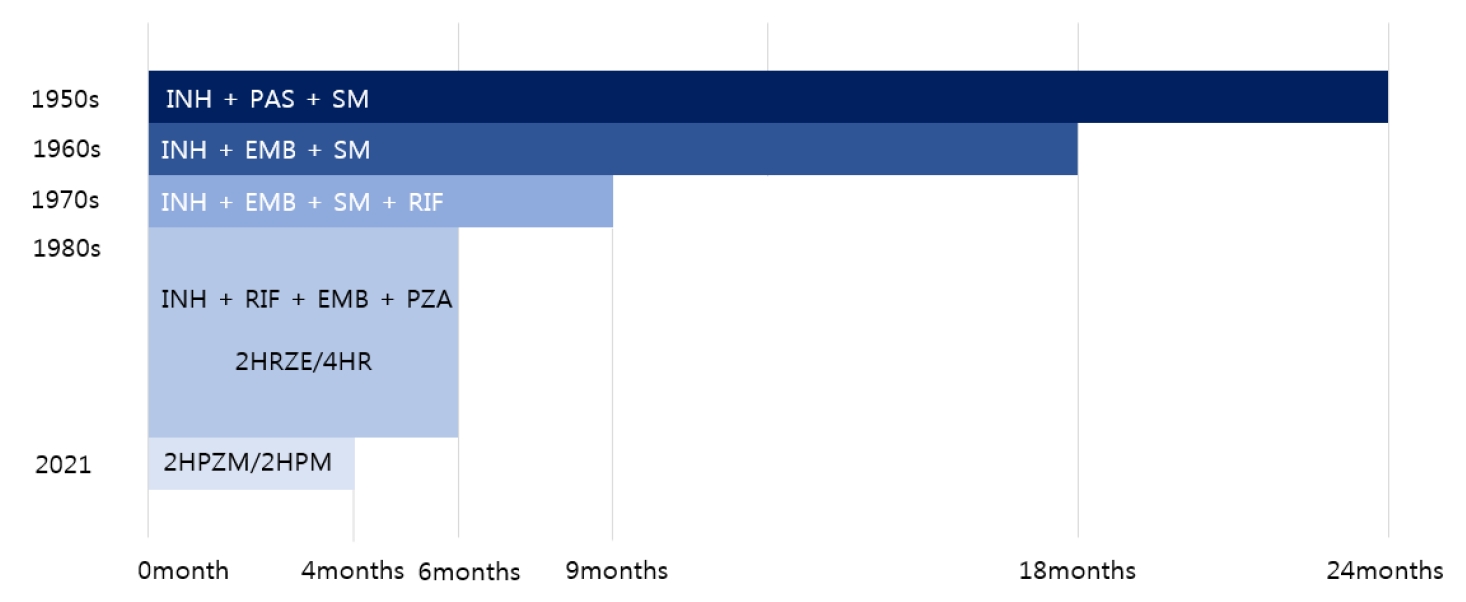
FigureВ 1.
TableВ 1.
Preferred drug regimens for microbiologically confirmed pulmonary tuberculosis caused by drug-susceptible organisms
Adopted from World Health Organization [26].
INH, isoniazid; RIF, rifampicin; EMB, ethambutol; PZA, pyrazinamide; H, isoniazid; R, rifampicin; Z, pyrazinamide; E, ethambutol; FDC, fixed dose combination.
TableВ 2.
Comparisons of current trials which aimed to shorten duration of TB treatment
| Regimen |
Intensive phase |
Continuation phase |
Per-protocol |
Modified ITT |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Drugs | Interval | Drugs | Interval | Favorable (%) | Unfavorable (%) | Differencea | Favorable (%) | Unfavorable (%) | Differencea | ||
| Control |
2 months |
4 months |
|||||||||
| INH, RIF, EMB, PZA | Daily | INH, RIF | Daily | ||||||||
| REMoxTB | Control | 92 | 8 | NA | 84 | 16 | NA | ||||
| 2 months | 2 months | ||||||||||
| INH Group | INH, RIF, MFX, PZA | Daily | INH, RIF, MFX | Daily | 85 | 15 | 6.1 (1.7-10.5) | 77 | 23 | 7.8 (2.7-13.0) | |
| EMB Group | MFX, RIF, EMB, PZA | Daily | MFX, RIF | Daily | 80 | 20 | 11.4 (6.7-16.1) | 76 | 24 | 9(3.8-13.0) | |
| OFLOTUB | Control | 88.7 | 11.3 | NA | 82.8 | 17.2 | NA | ||||
| GFX Group | 2 months | 2 months | 82.3 | 17.7 | 3.5 (-0.7 to 7.7) | 79 | 21 | 3.5 (-0.7 to 7.7) | |||
| INH, RIF, GFX, PZA | Daily | INH, RIF, GFX | Daily | ||||||||
| RIFAQUIN | Control | 95.1 | 4.9 | NA | 85.6 | 14.4 | NA | ||||
| 4M Group | 2 months | 2 months | 81.8 | 18.2 | 13.6 (7.0-20.2) | 73.1 | 26.9 | 13.1 (6.8-19.4) | |||
| MFX, RIF, EMB, PZA | Daily | MFX, RPT | Twice weekly | ||||||||
| 6M Group | 2 months | 4 months | 96.8 | 3.2 | -1.8 (-6.1 to 2.4) | 86.3 | 13.7 | 0.4 (-4.7 to 5.6) | |||
| MFX, RIF, EMB, PZA | Daily | MFX, RPT | Once weekly | ||||||||
| Microbiologically eligible population | Assessable population | ||||||||||
| Study 31/A5349 | Control | 85.4 | 14.6 | NA | 90.4 | 9.6 | NA | ||||
| RJPT Group | 2 months | 2 months | 82.3 | 17.7 | 3.0 (-0.6 to 6.6) | 85.8 | 14.2 | 4.4(1.2-7.7) | |||
| INH, RPT, PZA, EMB | Daily | INH, RPT | Daily | ||||||||
| RPT/MFX Group | INH, RPT, PZA, MFX | Daily | INH, RPT, MFX | Daily | 84.5 | 15.5 | 1.0 (-2.6 to 4.5) | 88.4 | 11.6 | 2.0 (-1.1 to 5.1) | |
Modified from Shin and Kwon [40].
ITT, intention-to-treat; NA, not applicable; INH, isoniazid; RIF, rifampicin; MFX, moxifloxacin; PZA, pyrazinamide; EMB, ethambutol; GFX, gatifloxacin; RPT, rifapentine.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print




