 |
 |
| Korean J Med > Volume 96(3); 2021 > Article |
|
Abstract
Helicobacter pylori (H. pylori) infection is one of the most common infectious diseases worldwide. Although its incidence is gradually decreasing, about half of the world's population still get infected. H. pylori infection is responsible for substantial gastrointestinal morbidity worldwide. It is the most common cause of gastric and duodenal ulcers as well as gastric cancer. Since the revision of the H. pylori Clinical Practice Guidelines in 2013, the eradication rate of H. pylori has gradually decreased with the use of classical triple therapy, wherein amoxicillin, clarithromycin, and proton pump inhibitors are administered, for 7 days. According to a nationwide randomized controlled study conducted by the Korean College of Helicobacter and Upper Gastrointestinal Research released in 2018, the intention-to-treat eradication rate was only 63.9%, which was due to increased antimicrobial resistance induced by the use of antibiotics, especially clarithromycin. The update of clinical practice guideline for treatment of H. pylori was developed based on evidence-based medicine by conducting a meta-analysis. The draft recommendations were finalized after expert consensus on three recommendations regarding the indication for treatment and eight recommendations on the treatment itself. These guidelines are designed to provide patients, nurses, medical school students, policymakers, and clinicians with clinical evidence to guide primary care and treatment of H. pylori infection. These may differ from current medical insurance standards and will be revised further, if necessary, based on research-based evidence.
헬리코박터 파일로리(Helicobacter pylori, H. pylori) 감염은 전 세계적으로 가장 흔한 감염성 질환의 하나로 지역마다 유병률이 다양하여 북유럽에서는 11%, 캐나다와 미국에서는 각각 23.1%, 30%로 낮으나 남미 72-82%, 나이지리아에서는 91%를 육박하며[1], 국내 유병률도 50% 내외로 높다[2]. H. pylori는 만성 위염부터 소화성 궤양, 위축성 위염 및 장상피화생, 위암까지 다양한 위장질환을 유발한다. 특히 한국, 일본, 중국을 포함한 극동 아시아는 H. pylori 감염률이 높으면서 위암 발생률이 높은데, 2017년 국내 암등록 통계 자료에 의하면 위암 표준화 발생률이 인구 100,000명당 32명으로 갑상선암을 제외한 전체 암종 중에 가장 높았다[3]. 일본의 경우, H. pylori 양성 위염의 제균 치료를 건강보험 급여로 인정하면서 사실상 H. pylori 양성인 모든 대상자를 제균 치료하도록 권장하고 있다[4]. 그러나 전 인구의 절반 정도에서 감염되어 있으나 일부(< 5%)에서만 위암이 발생하는 상황에서[5] H. pylori를 치료하기 위하여 여러 종류의 광범위 항생제를 투여하는 것에 대한 임상 근거가 확실치 않고, 높은 비용이 요구되며 항생제 내성 증가 가능성이 있어 위해(harm)에 비하여 확실한 이득(benefit)이 있다는 근거는 없다. 그러므로 국내 실정에 맞는 적절한 H. pylori 제균 치료의 적응증을 확립할 필요가 있다.
2013년에 발표된 H. pylori 진단 및 치료에 대한 임상 진료 지침은 국외의 진료 지침을 수용·개발하는 방법으로 국내 상황에 맞도록 제정하였으나, 그 개발 방법에 제한점이 있었다. 이번 개정안에서는 H. pylori 제균 치료가 필요한 환자 중 논란의 여지가 있던 철결핍성 빈혈, 위선종 제거 후, 위축성 위염 및 장상피화생에서 제균 치료가 필요한가에 대한 체계적 문헌고찰을 시행하여 그 근거를 생성하고자 하였다.
H. pylori 제균 치료는 적절한 항생제와 산분비억제제의 조합을 이용하며, 제균율이 80% 이상 되어야 한다[6]. 최근 일차 치료로 사용되어 온 표준 3제요법(양성자펌프억제제[proton pump inhibitors, PPIs] + amoxicillin + clarithromycin) 7일 치료는 제균율이 80% 미만으로 감소하였는데, 제균율 저하의 주요 원인은 clarithromycin 항생제 내성 때문이다[7]. Clarithromycin은 상기도 감염에 흔히 사용되는 광범위 항생제로서, 내성률이 15% 이상으로 높은 지역에서는 clarithromycin을 포함하는 3제 요법은 H. pylori 제균율이 낮아 권장되지 않는다[8]. 전 세계적으로 clarithromycin 내성률이 증가하고 있는데 북미 10%, 아시아 태평양 지역 17%, 유럽이 18%이며, 국내에서도 1995년 9%, 2003년 13.8%, 2005년 16.7%로 증가하는 추세이다[9-11]. 2018년 대한상부위장관·헬리코박터학회 주도 연구에서도 내성률이 17.8%였으나[2] 내성 검사를 시행하는 지역과 colony 수에 따라 30% 이상으로 측정되는 지역이 있어 clarithromycin 내성이 제균율 감소의 주 원인으로 뽑힌다[12]. 한편, metronidazole은 2000년대 초반까지 내성률이 60% 내외로 높은 양상을 보였으나[9], 2018년 대한상부위장관·헬리코박터학회 주도 연구에서는 29%로 낮아져 있었으며 내성이 있는 경우 기간을 늘리거나 높은 용량으로 사용 시 좋은 효과가 있다는 보고들이 있어 clarithromycin 내성률을 극복할 수 있는 대안이 될 수 있다[2].
표준 3제요법의 제균율이 감소하면서 다양한 약제 조합과 약제 투여 기간이 다른 제균 치료법이 소개되었다. 이번 진료 지침에서는 표준 3제요법 7일 치료의 대안으로서 다양한 치료법에 대한 임상 근거를 알아보기 위하여 체계적 문헌고찰과 메타분석을 시행하여 한국에 적절한 H. pylori 대상과 효과적 치료법을 제시하고자 한다.
본 진료 지침은 H. pylori 감염이 있는 성인 남녀를 대상으로, H. pylori 감염과 연관되어 감염과 연관된 증상이 있거나 연관된 질환을 가지고 있는 환자들을 대상으로 한다.
본 지침의 목적은 제균 치료가 필요한 임상 적응증을 정의하고, H. pylori 제균 약제 투여로 인한 부작용 및 내성률 발현은 최소화하면서 제균율을 극대화할 수 있는 적절한 일차 치료법 및 구제 치료법을 제시하여, 궁극적으로 H. pylori와 연관된 위염, 소화성 궤양 및 악성 위장관 질환 등 상부 위장관 질환을 치료하고 예방하는 것이다.
임상 진료 지침의 범위는 진료 지침 개발 그룹에서 명목 집단기법으로 patient intervention comparatives outcomes (PICO) 형식에 맞춘 핵심질문 도출을 통하여 결정하였고 그 구체적인 내용은 표에 정리하였다.
본 진료 지침은 H. pylori 감염을 진단하고 치료하는 내과 의사, 가정의학과 의사 및 일차 진료의 뿐만 아니라 약사, 간호사, 내시경 검사 인력 및 병리 기사 등 보건의료종사자가 임상에서 의사 결정을 수행할 때 사용할 수 있다. 또한 교육 목적으로 의과대학, 약학대학 및 간호대학 학생, 내과 및 가정의학과 수련의, 간호사 및 의료 기사도 사용할 수 있다. 또한 H. pylori 연관 질환의 진료 및 치료가 필요한 환자 및 일반인도 이용할 수 있으며, H. pylori 치료에 대한 임상 근거를 바탕으로 최적의 지침 개발을 통하여 건강 보험 적용 등에 대한 정책 결정의 표준을 제시하고자 한다.
임상 진료 지침 운영위원회는 대한상부위장관·헬리코박터학회 회장 및 임원진을 중심으로 구성하여 진료 지침 개발과 관련된 개발전략을 수립하고 실무위원장을 선임하였으며, 사업과 연관된 예산을 검토하고 승인하였다. 본 지침은 대한상부위장관·헬리코박터학회에서 모든 예산을 지원받았으나 이외 별도의 재정 후원은 없었으며, 학회의 재정 후원이 지침 개발에 영향을 주지 않았다. 지침 개발 실무팀은 H. pylori 관련 전문가인 소화기내과 의사를 중심으로 지역적 안배를 고려하여 위원장(정혜경), 간사(강승주) 및 위원(양효준, 박선영, 신철민, 김성은, 임현철, 김지현, 남수연, 신운건, 박재명, 최일주)으로 구성하였고, 근거의 선택, 검색 및 등급화를 시행하고 최종 권고안을 도출하였다. 지침 개발의 방법론 확립을 위하여 방법론 전문가 2명(신인순, 최미영)과 대한의학회 임상 진료 지침 전문위원인 김수영 교수와 함께 문헌검색 및 문헌의 질평가, 메타분석의 실제, 임상 진료 지침 근거와 권고 등급 설정, 전문가 합의에 관한 4회의 워크샵을 시행하였다. 본 진료 지침은 대한소화기학회, 대한병리학회 및 대한임상미생물학회 등 위원을 포함한 다학제 위원회를 구성하여 지침 개발 과정을 수행하였다.
진료 지침 적용 대상인 환자 혹은 일반인의 경험과 기대, 선호도를 반영하기 위하여 위장 질환과 연관된 최대 인터넷 커뮤니티를 통하여 구조화된 설문지로 H. pylori 치료에 관한 설문조사를 일반인들에게 실시하였다. 총 233명이 응답하였고, 응답자의 64.4%가 성인 여성이었으며 H. pylori 양성인 경우가 57.5%였다. H. pylori 양성인 경우 치료 의향이 있는 경우가 86.7%였고, 이 중 제균 치료를 하고 싶은 이유는 위암 예방 목적 44.6%, 위장 증상 호전 28.8%, 타인에게 전염 우려가 9.9%였다. H. pylori 제균 치료에 대하여 가장 걱정스러운 점은 약제 부작용이 80.3%였다. 이러한 점에 근거하여 H. pylori로 인한 위암 발생의 전구 병변인 위축성 위염과 장상피화생이 제균 치료의 적응이 되는지 및 약물 치료 부작용 부분을 개발 주제에 추가하였다.
최종 도출된 권고안을 바탕으로 실무위원회에서 초안을 작성하여 실행위원회 및 운영위원회에서 내부 검토를 수행하였고, 환자의 가치와 선호도, 촉진 요인과 장애요인에 대하여 비임상전문가를 포함한 외부 검토를 수행하였다. 검토 과정에서 도출된 의견 및 검토 의견의 반영 결과를 정리하였다.
본 지침과 Supplementary material들은 대한상부위장관·헬리코박터학회(http://www.hpylori.or.kr)와 대한내과학회(http://www.kaim.or.kr)의 웹사이트에서 지침안을 볼 수 있도록 하였다. 또한 본 임상 진료 지침은 국내외 H. pylori 제균 치료와 연관된 주제에 대한 메타분석을 시행하여 아시아 및 전 세계적 근거를 요약한 것으로 학회지에 게재하여 지침안을 볼 수 있도록 하였고 학회 트위터, 페이스북 등 소셜미디어를 통하여 이용이 가능하도록 하였다.
최근 국내 H. pylori 감염 및 관련된 위장관 질환의 역학과 항생제 내성률이 급격히 변화하는 추세이기 때문에 최신의 근거가 필요하여 이번 지침의 개정은 신규 직접(de novo) 개발을 시행하였다.
핵심질문을 도출하기 위하여 지침 개발 실무팀에서 기존 진료 지침을 검색하고 대면회의를 통하여 H. pylori 치료의 대상이 되는 적응증과 치료에 대한 주제를 선정하였다. 이후 담당 실무 위원을 정한 뒤, 다음 대면회의에서 각 주제에 타당한 PICO를 정한 뒤 토론 과정을 통해 최종 12개를 선정하였다. 주제 선정에 있어 환자선호도를 반영하기 위하여 국내 위장관 질환 관련 인터넷 커뮤니티에서 H. pylori 감염 치료와 연관된 일반인의 의견, 지침의 범위 등에 관한 조사를 실시하여, 위암의 전구 병변, 즉, 위선종 및 위축성 위염/장상피화생에서 H. pylori 제균 치료의 효과와 H. pylori 제균 치료 부작용에 대한 권고안을 선정하였다.
지침의 근거가 되는 연구 문헌을 검색하기 위하여 각 주제별 체계적 문헌고찰을 실시하였다. 한국보건의료원 최미영 박사와 각 주제별 실무위원이 적절한 검색어를 선정하여 Ovid-MEDLINE, EMBASE, Cochrane Library, KoreaMed 및 KMBASE에서 2018년 8월까지 출판된 문헌들을 주제별로 검색을 실시하였고(Fig. 1) 검색의 공통된 선정기준은 1) 성인을 대상으로 한 연구, 2) 영어 혹은 한국어로 기술된 문헌, 3) 관찰 연구, 무작위 대조군 연구(randomized controlled trial, RCT), 체계적 문헌고찰 및 메타분석, 4) 2008년부터 2018년까지 발행된 연구, 5) 적절한 결과(제균율, 부작용 빈도, 증상 호전, 위암 발생 등)가 보고된 경우로 하였다. 공통된 제외 기준은 1) 소아나 청소년을 대상으로 한 연구, 2) 적절한 결과(제균율, 부작용 빈도, 증상 호전, 위암 발생 등)가 보고되지 않은 연구, 3) 영어나 한국어 이외의 언어로 기술된 문헌, 4) 중복으로 게재된 경우(동일 내용으로 다른 저널에 게재 혹은 출판 형태만 차이가 있는 경우), 5) 원문 확보가 불가능한 경우, 6) 전문가 의견이나 증례보고, 종설, 진료 지침 등이었다. 각 주제별로 위원 2인이 한 조가 되어 근거 문헌을 선택하였고, 포함 여부가 불분명할 때에는 지침개발그룹 회의에서 포함여부를 논의하였다. 각 주제별로 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)에서 제시한 문헌 선택 흐름도를 사용하여 문헌 선택 과정을 정리하였다(Fig. 1).
체계적 문헌고찰을 통하여 최종 선택된 문헌을 바탕으로 사전에 작성된 근거표에 정리하였고, 이를 바탕으로 메타분석을 시행하였다. 근거 수준과 권고 강도(strength of recommendation)는 The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) 방법론을 이용하였다(Table 1) [15]. 근거 수준은 4등급으로 세분하였는데, 연구 디자인과 근거의 질을 주로 평가하였고, 비뚤림 위험, 일관성, 직접성, 정밀성, 출판 비뚤림 등을 고려하였다. 권고 강도는 강한 권고, 약한 권고, 권고하지 않음, 미결정의 4단계로 분류하였다. 권고 강도는 대상자에게 해당 중재를 시행하였을 때 위해보다 이득이 더 클 것으로 혹은 작을 것으로 확신하는 정도를 의미하며, 권고 결정 고려요소로는 근거수준, 효과 크기(이득과 위해의 정도 비교), 환자의 선호도와 가치, 자원 이용을 종합적으로 판단하였다[16]. 이 지침에서 제시되는 권고문들은 표 2에 정리되어 있다.
자원 및 경제성 근거를 활용하기 위하여 문헌 검색을 실시하였고, 환자선호도를 반영하기 위하여 비용에 대한 설문을 실시하였다. H. pylori 제균 치료는 항생제 및 PPI를 7일-14일 단기간 조합으로 사용하는데, 각 치료 방법 간의 비용의 차이는 크지 않다. 설문조사에서 환자에게 H. pylori 제균 치료에 드는 약제 비용이 부담되는지에 대하여 질문하였는데, 응답자의 8.2%에서만 비용이 부담이 된다고 응답하였다. H. pylori 맞춤 제균 치료법(tailored therapy based on antibiotics-susceptibility test)은 항생제 내성 여부를 제균 치료 전에 검사하는 방법으로 주로 clarithromycin 내성 검사를 시행하는데, 고비용이 드는 검사 방법이나 제균율이 75.3% 이하로 낮아지면 오히려 맞춤 치료가 비용-효과면에서 우월하다는 보고가 있다[17,18].
핵심질문별 권고안의 합의를 위하여 합의 방법에 대한 워크샵을 실시한 후 수정 델파이 방법으로 합의안을 도출하였다. 1차 합의를 위하여 H. pylori 질환의 전문가 44명을 초대하였고 30명이 참여에 동의하여 이메일을 통해 조사하였다. 1차 설문은 각 권고안과 그 근거를 제시한 자료에 대하여 동의 정도를 묻는 9-Likert scale의 자가 보고형 설문지를 이용하여 조사하였다. 응답 척도는 1점이 ‘전혀 동의하지 않는다’에서 9점 ‘매우 동의한다’까지이며, 7-9점(고합의)의 비율이 2/3 이상이면서 비동의가 없는 경우를 동의로 간주하였다. 1차 합의에서 총 12개의 권고안에 대하여 8개가 합의되었고, 치료 적응증에서 빈혈 및 위축성 위염/장상피화생, 치료 방법에서 표준 3제요법, 순차 치료가 동의에 이르지 못하여, 개발위원회에서 권고안을 수정하여 대면 합의로 2차 투표를 실시하였다(2019년 12월 14일). 수정된 2차 권고안의 근거에 대한 발표 후 무기명으로 2차 투표를 실시하였으며, 위축성 위염/장상피화생에 대한 권고안은 23명 중 48%의 찬성으로 기각되었고 나머지 권고안은 통과되어 최종적으로 11개의 권고안이 채택되었다.
헬리코박터 파일로리 제균 치료는 원인 미상의 철결핍성 빈혈 환자에서 혈색소를 증가시키는가?
지침 1. 헬리코박터 감염 제균 치료는 원인 미상의 철결핍성 빈혈을 가진 성인의 일부에서 도움이 될 수 있다.
권고 강도: 약함
근거 수준: 매우 낮음
전문가 합의: 76.0% (2차), 56.7% (1차)
빈혈은 유병률이 높고 다양한 전신 증상을 유발할 수 있는 질환으로 대부분이 철결핍성 빈혈이다[19,20]. 전 세계적으로 빈혈의 유병률은 24.8% (95% confidence interval [CI] 22.9-26.7%)로 약 16억 2천만 명(95% CI 15억 명-17억 4천만명)에 해당하며[19], 미취학 어린이와 여성에서 호발한다. H. pylori는 만성 위염, 소화성 궤양 및 악성 질환 등 다양한 위장 질환을 유발하고, 특히 H. pylori 감염으로 인한 만성 위염은 철분 흡수에 반드시 필요한 위산 분비와 위 내 아스코르브산을 감소시킨다[6,21].
H. pylori와 철결핍성 빈혈과의 관련성에 대한 근거는 아래의 연구 결과에 토대를 두고있다. 최근 발표된 메타분석에서 H. pylori 감염이 있는 군은 없는 군에 비하여 철결핍성 빈혈이 동반될 교차비(odd ratio [OR])가 1.72 (95% CI 1.23-2.42)로 높았다[22]. 그러나 메타분석에 포함된 대부분의 연구가 소아와 청소년을 대상으로 한 연구였으며, 성인만을 대상으로 한 하위분석에서는 OR 1.70 (95% CI 1.01-2.85)으로 통계적인 유의성은 관찰되었으나 심한 이질성(heterogeneity)을 보였다[19]. 철결핍성 빈혈에서 H. pylori 제균 치료 후 빈혈이 교정되는가에 대한 연구는 많지 않고 이질성이 높으며, 연구대상자가 소아이거나 청소년, 여성(임신 vs. 비임신)과 같은 특정군을 대상으로 한 연구여서 전체 성인에게 일반화하기 어려운 점이 있다. 또한, 제균 치료 후 빈혈의 호전 정도를 보는 연구 종료점이 혈청 ferritin이나 혈색소의 정량적 측정, 빈혈의 회복과 같은 정성적 측정 등 연구마다 다양하였다. 소아, 청소년 및 성인이 모두 포함되고 연구 종료점을 혈청 ferritin 상승으로 설정한 7개의 무작위 전향적 환자-대조군 연구를 바탕으로 한 메타분석에서 혈청 ferritin 상승의 표준화 평균 차이(standardized mean difference, SMD)는 0.53 (95% CI 0.21-0.85)으로 의미 있는 상승을 보였으나 혈색소 상승의 SMD는 0.36 (95% CI -0.07 to 0.78)으로 유의한 차이가 없었다[22].
본 지침에서 선정한 핵심질문에 해당하는 성인 철결핍성 빈혈 환자에서 H. pylori 제균 치료 효과에 대한 연구는 매우 제한적이다. H. pylori 양성 만성 위염이 있으면서 철결핍성 빈혈이 있는 성인에서 제균 치료가 일반적인 빈혈 치료에 추가적인 빈혈 개선 효과가 있는지를 본 비무작위 대조군 연구가 있고[23], H. pylori 감염이 있으면서 ferritin이 낮은 성인을 대상으로 제균 후 혈청 ferritin 상승을 본 전향적 관찰 연구가 있으나 연구대상자 수가 적었다는 제한점이 있다[24]. 하지만 이 연구에서 H. pylori 감염군에서 유의하게 철결핍성 빈혈이 호발하였고, 제균 치료 후 혈청 ferritin이 상승하거나 빈혈이 소실되었다[24]. H. pylori 감염이 있는 88명의 환자에서 제균 치료 후 철결핍성 빈혈이 38.1%에서 소실되었고, 특히 남성과 폐경 후 여성에서 폐경 전 여성에 비하여 유의하게 빈혈 소실율이 높았다(75% vs. 23%, p< 0.01) [25]. 근거 수준이 매우 낮음에도 불구하고, 단기간 H. pylori 제균 치료로 장기적으로 빈혈이 교정되는 이득의 가능성이 있고 위해가 크지 않아 ‘약한 권고’로 결정하였다.
위선종의 내시경 절제 후 헬리코박터 제균 치료는 이시성 위암의 발생 예방에 도움이 되는가?
지침 2. 헬리코박터 양성 위선종 환자에서 내시경 절제 후 이시성 위암의 발생 예방을 위해 헬리코박터 제균 치료를 권고할 수 있다.
권고 강도: 약함
근거 수준: 낮음
전문가 합의: 80.0% (1차)
많은 연구들에 따르면 조기 위암의 내시경 절제술을 받은 환자에서 H. pylori 제균 치료 후 이시성 위암의 발생률이 감소하였다[26-28]. 따라서 조기 위암의 내시경 절제술 후 이시성 위암의 발생 감소를 위해 제균 치료는 시행되어야 한다. 그러나 위선종의 내시경 절제 후의 H. pylori 제균 치료에 대해서는 명확한 지침이 없다. 지금까지 조기 위암 및 선종을 포함한 위종양의 내시경 절제술 후 이시성 위암의 발생을 예방하기 위한 제균 치료에 관한 두 개의 RCT가 있었다[26,28]. 위선종의 내시경 절제 후 이시성 병변의 예방을 위한 H. pylori 제균 치료에 관한 후향적 연구는 3개가 있었는데, 이들 모두 국내에서 수행된 연구들이다(Supplementary Table 1) [29-31]. 이들 연구들에 따르면 H. pylori 제균 치료 후 이시성 위암의 발생률은 제균되지 않은 군보다 낮았다(3.2% vs. 4.9%; 7.7% vs. 14.3%; 7.8% vs. 10.8%; 8.2% vs. 19.4%; 4.7% vs. 11.4%). 위의 5건의 연구를 포함한 메타분석에서 위선종의 내시경 절제 후 이시성 위암의 발생 예방에 대한 제균 치료의 효과는 통계적으로 유의하였다(OR 0.55; 95% CI 0.34-0.92) (Fig. 2). 이는 H. pylori 제균 치료는 위선종의 내시경 절제 후 이시성 위암의 발생을 예방하는 데에 도움이 됨을 시사한다. 따라서 H. pylori 양성 위선종 환자에서 내시경 절제 후 H. pylori 제균 치료가 권고된다. 하지만, 위선종 환자만을 대상으로 한 RCT가 아직 없어 이에 대한 연구가 필요하다.
헬리코박터 파일로리 양성인 기능성 소화불량증 환자에서 제균 치료는 장기적인 증상 개선에 도움이 되는가?
지침 3. 헬리코박터 제균 치료는 기능성 소화불량증의 장기적인 증상 완화에 도움이 되기 때문에 권고할 수 있다.
권고 강도: 약함
근거 수준: 높음
전문가 합의: 70.0% (1차)
RCT들의 메타분석에서 기능성 소화불량증 환자의 H. pylori를 제균하였을 때, 단기간(3개월) 추적 관찰 시 증상 개선 효과는 유의하지 않았지만, 장기간(6-12개월) 추적 관찰에서 유의하게 증상이 개선되었다[32,33]. 이 결과를 바탕으로 유럽의 Maastricht V 가이드라인과 미국과 캐나다 가이드라인에서는 소화불량증의 일차적인 치료로 H. pylori 제균 치료를 강력히 권고하고 있다[34,35].
본 지침에서는 소화불량증 환자에서 H. pylori 제균 치료의 장기 효과를 평가하기 위하여 1997년 1월부터 2017년 12월까지 18개의 RCT를 선택하여 메타분석을 시행하였다[36-53]. 총 18개 연구, 4,672명을 대상으로 시행한 메타분석에서 대조군이 제균 치료군에 비해 소화불량 증상이 지속될 위험도비(risk ratio, RR)가 1.18 (95% CI 1.07-1.31)로 통계적으로 유의하였으나, 치료에 필요한 환자 수(number needed to treat, NNT)가 14로 그 효과가 월등하지 않았고 연구간의 이질성은 중등도였다(I2 = 34%) (Supplementary Table 2; Supplementary Fig. 1) [54].
연구 간에 이질성이 있기 때문에 지역에 따라 하위 집단 분석을 시행하여 5개의 아시아 지역에서 나온 논문들과 13개의 아시아 외의 지역에서 나온 논문들을 분석하였다. 아시아 외 지역에서 나온 논문들을 분석하면 제균 치료는 유의한 이질성 없이 소화불량 증상의 개선 가능성을 유의하게 높였다(RR 1.22; 95% CI 1.08-1.38; I2 = 33%). 하지만 아시아에서 나온 연구들을 분석한 결과 제균요법이 소화불량 증상 개선에 미치는 영향은 유의하게 높지 않았다(RR 1.10; 95% CI 0.92-1.31; I2 = 32%).
정리하면, 기능성 소화불량증에서 H. pylori 제균 치료는 통계적으로 유의하게 증상을 개선시켰으나 14명의 소화불량증 환자들 중 1명에서 제균 치료로 증상이 호전되는 정도여서(NNT = 14) 임상 효과가 크다고 보기 힘들고, 아시아 지역에서 수행된 연구들의 하위 분석 결과는 통계적으로 유의하지 않았다. H. pylori 유병률을 전 세계적으로 추정한 논문에 따르면 국내 H. pylori의 유병률은 54% (95% CI 50.1-57.8%)로 추정된다[55]. H. pylori 유병률이 높은 지역에서는 제균요법과 관련된 비용과 부작용, 내성 균주의 출현 위험 및 재감염 위험이 유병률이 낮은 지역 보다는 높을 것으로 생각되어 이번 지침에서는 소화불량증에서 H. pylori 제균 치료는 근거가 높음에도 불구하고 약한 권고를 하기로 결정하였다. 추후 국내를 포함하여 H. pylori 유병률이 높은 지역의 기능성 소화불량증 환자에서 제균 치료의 비용 효과 분석을 포함하는 RCT가 필요하다.
헬리코박터 파일로리 양성이면서 위축성 위염, 장상피화생이 있는 경우 제균 치료를 하는 것이 위암 예방에 효과가 있는가?
H. pylori 제균 치료는 위암의 발생 위험을 줄인다고 알려져 있다. 하지만, 위축성 위염이나 장상피화생과 같이 전암성 병변을 가진 환자에서 제균 치료가 위암의 예방에 효과가 있는지에 대해서는 아직 논란의 여지가 있다.
최근에 발표된 두 개의 메타분석 결과에 따르면 전암성 병변이 전혀 없는 경우나 위축성 위염이 있는 경우에 H. pylori 제균 치료가 향후 위암의 발생 위험을 줄이지만 장상피화생이나 위선종이 있는 경우에는 위암의 발생 위험을 줄이지 못하였다[56,57]. 또한 제균 치료의 효과는 위축성 위염의 정도에 의해 영향을 받아 광범위한 위축성 위염을 가진 환자보다 경증의 위축을 가지고 있는 환자에서 H. pylori 제균 치료가 위암을 예방하는 데 더 효과적이었다[58]. 이 연구를 기반으로 Maastricht V 지침에서는 위축성 위염이나 장상피화생의 발생 전에 제균 치료를 함으로써 위암을 효과적으로 줄일 수 있다고 권고하고 있다[34].
하지만, 위에서 언급된 두 개의 메타분석은 일반인에 대한 연구뿐만 아니라, 조기 위암으로 내시경 절제술을 받은 환자에서 제균 치료 후 이시성 위암이 발생하였는지를 본 연구도 포함하고 있다. 제균 치료의 효과는 일반인군과 조기 위암으로 내시경 치료를 받은 고위험군에서 다를 수 있다. 또한 두 메타분석에 포함되지 않은 일반인을 대상으로 한 중국의 대규모 코호트 연구에 따르면 H. pylori 제균 치료는 장상피화생이 있거나 위선종이 있던 환자에서도 위암의 위험도를 줄였다[59]. 이 연구는 일반인을 대상으로 하였을 때는 장상피화생이 있어도 제균 치료가 위암 예방 가능성이 있음을 시사한다.
본 지침에서 일반 인구 집단을 대상으로 한 무작위 대조 연구만을 선택하여 메타분석을 시행한 결과, 기존 연구와 같이 H. pylori 제균 치료는 유의하게 위암의 발생을 감소시켰지만(Fig. 3), 위축성 위염이나 장상피화생을 가진 대상자만을 포함한 하위 분석에서는 제균 치료가 위암의 예방에 효과가 없었으며, 위축성 위염이나 장상피화생이 없는 대상자를 포함한 2개의 연구에서도 유의한 위암 예방 효과를 입증하지 못하였다(Supplementary Fig. 2). 그러나 후자의 경우, 위암의 발생건수가 작아 정확한 결론을 도출하는 데 제한점이 있다. 이 결과를 근거로 전문가 합의도출을 실시한 결과, 1차 이메일 설문에서 48.0%가 동의하였고, 2차 대면 합의에서는 63.3%만이 동의하였다. 즉, 위축성 위염이나 장상피화생에서 H. pylori 제균 치료를 권고하기에는 확실한 근거나 전문가 합의가 없어 이에 대한 권고를 내는 것은 이번 지침에서 보류하였고 추후 위축성 위염이나 장상피화생이 있는 일반 인구 집단에서 위암 예방에 H. pylori 제균 치료가 도움을 주는지에 대한 연구 결과가 축적된 후 재논의가 필요하다. 이상에서 제시된 H. pylori 제균 치료의 적응증들과 기존의 가이드라인에서 권고되었던 적응증들인 소화성 궤양, 변연부 B세포 림프종, 조기 위암의 내시경 절제술 후, 위암 가족력이 있는 경우, 만성 특발성 혈소판 감소증, 소화성 궤양의 병력이 있는 환자에서 장기간 저용량 아스피린 복용하는 경우의 적응증들은 표 3에 정리되어 있다.
H. pylori 제균 치료를 처음 시행하는 환자에서 1) 표준 3제요법, 2) bismuth를 포함하지 않는 4제요법(순차 치료, 동시 치료 등), 3) clarithromycin 내성 검사 후 표준 3제요법 선택, 4) 일부 환자에서 bismuth 포함 4제요법을 사용할 수 있다.
표준 3제요법은 헬리코박터 파일로리 감염의 1차 제균 치료로 사용될 수 있는가?
지침 4. 1차 제균 치료로 표준 3제요법(양성자펌프억제제 표준 용량, amoxicillin 1 g, clarithromycin 500 mg 하루 2회) 14일 치료를 권고한다.
권고 강도: 강함
근거 수준: 중간
전문가 합의: 77.0% (2차), 63.3% (1차)
1차 제균요법의 적절한 선택을 위해서는 지역의 항생제 내성률과 약물 치료에 따른 제균율을 고려해야 한다. 제균 치료의 성공 여부에는 환자의 순응도, 항생제의 항균력에 영향을 줄 수 있는 PPI의 산분비 억제능 등 다양한 인자들이 관련되나, 가장 중요한 요인은 항생제 내성, 특히 clarithromycin 내성 유무이다. 한국에서 clarithromycin 내성률은 지난 10년 동안 점차 증가해왔으며, 최근의 연구에서는 17.8-31.0%로 높다[11,60]. 한국 내에서도 지역에 따라 clarithromycin 내성률은 차이를 보이고 있는데, 전국 항생제 내성률 조사에서 서울과 충청도 지역은 15% 미만이었으나 이 두 지역을 제외한 지역에서는 15% 이상의 내성률을 보였다[2]. 제균율을 기준으로 할 때, H. pylori 제균 치료에서 ‘intention-to-treat (ITT)’ 제균율이 적어도 80% 이상이 되어야 초치료 요법으로서 추천할 수 있다[61].
본 지침에서는 한국에서 최근 10년간의 표준 3제요법의 제균율을 구하기 위해 2007년 이후 표준 3제요법을 이용한 RCT들을 대상으로 체계적 문헌 검색을 실시하였다. 사전에 정해진 선택 기준과 제외 기준에 따라 총 26개의 연구들이 선택되었다(Supplementary Table 3) [62-87]. 이 연구들로부터 도출된 표준 3제요법의 ITT 제균율은 71.6% (95% CI 69.9-73.3%)였고, per protocol (PP) 제균율은 79.6% (95% CI 76.6-82.2%)였다(Fig. 4). 위의 연구들을 기간별로 나누면 2007년부터 2011년까지 ITT 제균율은 72.3% (95% CI 71.2-74.4%), 2012년부터 2016년까지는 70.3% (95% CI 68.4-72.1%)로 제균율이 감소하였다. 결과를 종합해보면 표준 3제요법의 제균율은 유의하게 감소하여 ITT 제균율은 71.6%로 초치료로 사용하기에 부적절하였다. 이는 2018년 대한상부위장관·헬리코박터학회에서 시행한 전향적 무작위 연구와 유사한 결과였다[88]. 따라서 7일 표준 3제요법의 ITT 제균율이 80%보다 낮아 초치료로 사용하기 위해서는 clarithromycin 감수성 검사를 도입하거나 다른 제균 치료를 선택할 것을 고려해야 한다.
본 지침에서 치료 기간에 따른 표준 3제요법의 제균율을 보기 위하여 7일, 10일, 14일 치료의 제균율에 대한 하위 분석을 시행하였다. 7일 표준 3제요법의 ITT 제균율은 70.0% (95% CI 68.5-71.4%), 10일 치료 73.7% (95% CI 69.8-7.2%), 14일 치료 78.1% (95% CI 75.2-0.7%)로, 14일 치료의 제균율이 7일이나 10일 치료에 비해 유의하게 높았으나(각각 p< 0.01), 7일과 10일 치료의 제균율 간에는 유의한 차이가 없었다(Supplementary Fig. 3).
같은 주제를 다룬 2017년 네트워크 메타분석에서도 유사한 결과를 보였으며, 7일, 10일, 14일 치료의 ITT 제균율은 각각 71.1% (95% CI 68.3-73.7%), 67.0% (95% CI 60.0-73.4%), 76.4% (95% CI 73.3-79.2%)였다[89]. 또한 2019년에 발표된 전국 다기관 연구 결과, 7일 표준 3제요법의 ITT 제균율은 63.9%, PP 제균율은 71.4%였다[90]. 상기의 분석과 근거에 의하면 clarithromycin 내성 검사 없이 표준 3제요법을 1차 제균 치료로 사용할 때는 14일 요법이 권장된다.
순차 치료는 헬리코박터 파일로리 감염의 1차 제균 치료로 사용될 수 있는가?
지침 5. 1차 제균 치료로 10일 순차 치료(양성자펌프억제제 표준 용량과 amoxicillin 1 g 하루 2회 5일간 사용 후 양성자펌프억제제 표준 용량, clarithromycin 500 mg, metronidazole 500 mg 하루 2회 5일간)를 권고한다.
권고 강도: 강함
근거 수준: 높음
전문가 합의: 70.0% (2차), 63.3% (1차)
최근 여러 외국 가이드라인에서 clarithromycin 내성률이 15% 이상인 지역에서는 bismuth를 포함하지 않는 4제요법(non-bismuth quadruple therapy)인 순차 치료나 동시 치료, 또는 bismuth 4제요법을 1차 치료로 권장한다[91,92]. Bismuth를 포함하지 않는 4제요법은 PPI와 함께 amoxicillin, clarithromycin, metronidazole의 4가지 약제를 동시에 사용하되 그 방법마다 개별 항생제의 사용 기간이 다르다. 순차 치료는 PPI와 amoxicillin을 초기 5일간 사용한 후, 6일부터 10일까지 5일간 PPI, clarithromycin과 metronidazole을 투여하는 방법이다.
1차 제균 치료로서 순차 치료에 대한 효과를 확인하기 위하여 24개의 RCT를 대상으로 메타분석을 시행하였다(N = 5,070) (Supplementary Table 4) [72,74-77,87,93-110]. 표준 3제요법과 비교한 RCT 20개, bismuth 포함 4제요법과 비교한 연구 2개, 병합 치료(hybrid therapy)와 비교한 연구 2개가 포함되었으며, 동시 치료와의 비교 연구는 동시 치료 부분에서 기술하였다.
표준 3제요법과 10일 순차 치료의 치료 성적을 비교한 메타분석에서 10일 순차 치료가 표준 3제 치료에 비해 ITT 분석에서는 37% (95% CI 1.21-1.52), PP 분석에서는 60% (95% CI 1.40-1.93) 제균율이 높았다. 이를 표준 3제요법의 치료 기간으로 나누어 분석해 보면 10일 순차 치료는 7일과 10일 표준 3제요법에 비하여 유의하게 제균율이 높았으나, 14일 표준 3제요법과는 유사한 제균율을 보였다(Fig. 5).
20개 연구의 10일 순차 치료의 제균율(pooled eradication rates)을 종합해보면 ITT 분석 80.3%, PP 분석 86.6%였으며, 이중 7개의 국내 연구만을 분석하였을 때에는 ITT 분석 78.6%, PP 분석 87.5%였다.
10일 순차 치료와 bismuth 4제요법을 비교한 2개의 RCT 연구의 메타분석에서 두 치료법의 제균율은 차이가 없었으나(RR 0.79; 95% CI 0.47-1.32 in ITT analysis; RR 0.63; 95% CI 0.27-1.49 in PP analysis), 포함된 환자수가 적고 국내 연구가 포함되지 않았다는 제한점이 있다(Supplementary Fig. 4).
10일 순차 치료와 병합 요법을 비교한 2개의 RCT 연구의 메타분석에서 10일 순차 치료가 병합 요법에 비해 제균율이 낮았으나 (RR 0.36; 95% CI 0.23-0.58 in ITT analysis; RR 0.18; 95% CI 0.10-0.35 in PP analysis), 포함된 환자수가 적고 국내 연구가 포함되지 않았다는 제한점이 있다.
요약하면, 1차 제균 치료로서 10일 순차 치료의 제균율은 표준 3제요법의 제균율에 비해 높았으며, 하위 분석에서 7일과 10일 표준 3제요법에 비하여 순차 치료가 높은 제균율을 보였으나 14일 표준 3제요법과는 유사하였다. Bismuth 4제요법과 병합 요법과의 비교 분석은 RCT 수가 적어 결론을 내리기 어렵다. 그러므로 국내에서 clarithromycin 내성이 증가함에 따라 내성 검사 없이 1차 제균 치료를 하는 경우 10일 순차 치료를 권고한다.
동시 치료는 헬리코박터 파일로리 감염의 1차 제균 치료로 사용될 수 있는가?
지침 6. 1차 제균 치료로 10일 동시 치료 (양성자펌프억제제 표준 용량, clarithromycin 500 mg, amoxicillin 1 g, metronidazole 500 mg 하루 2회)를 권고한다.
권고 강도: 강함
근거 수준: 높음
전문가 합의: 80.0% (1차)
동시 치료는 표준 3제요법의 제균율 감소를 극복하기 위하여 사용되는 bismuth 미포함 4제요법의 하나로 PPI와 함께 amoxicillin, clarithromycin, metronidazole을 10일간 동시에 투여하는 방법이다.
1차 제균 치료로 동시 치료의 효과를 보기 위하여 동시 치료를 포함한 RCT를 추출하였고, 5일, 7일 동시 치료 단기요법은 제외하였다. 총 26개의 RCT들이 최종적으로 분석에 포함되었다(Supplementary Table 5) [81,86,108,111-130]. 21개의 10일 동시 치료, 6개의 14일 동시 치료가 포함되었고, 1개의 RCT는 10일 동시 치료와 14일 동시 치료를 모두 포함하고 있었다. 10일 동시 치료의 제균율은 ITT 분석 85%, PP 분석 91%였고, 14일 동시 치료의 제균율은 ITT 분석 86%, PP 분석 94%로 표준 3제요법보다 높았고, 10일 동시 치료와 14일 동시 치료의 제균율은 차이를 보이지 않았다. 국내 연구에 대한 하위 분석에서 10일 동시 치료 제균율은 ITT 분석 84%, PP 분석 92%, 14일 동시 치료 제균율은 ITT 분석 79%, PP 분석 94%였으며, 투여 기간에 따른 제균율의 차이는 없었다(Supplementary Table 6).
동시 치료와 다른 치료법의 제균율을 표준화된 기준으로 근거의 질을 평가하기 위하여 GradePro (www.gradepro.org, McMaster University and Evidence Prime Inc., Hamilton, Ontario, Canada)를 이용하였다. 10일 동시 치료는 순차 치료에 비해서 근소하게 제균율이 높았고 10일/14일 표준 3제요법에 비해서는 17% 높은 제균율을 보였으며, 이에 대한 근거 수준은 높았다. Bismuth 4제요법, 병합 요법과는 제균율의 차이를 보이지 않았으며, 근거 수준은 각각 중등도, 높음으로 평가하였다.
검색 과정을 통해 10일 동시 치료와 다른 치료법을 비교한 21개의 RCT들이 선정되었다. 8개의 RCT에서 10일 동시 치료와 10일/14일 표준 3제요법의 제균율을 비교하였다. 10일 동시 치료 제균율은 10일/14일 표준 3제요법의 제균율보다 유의하게 높았다(RR 1.17; 95% CI 1.05-1.30 in ITT analysis; RR 1.15; 95% CI 1.06-1.25 in PP analysis) (Fig. 6).
13개의 RCT에서 10일 동시 치료와 10일 순차 치료를 비교하였다. 10일 동시 치료 제균율이 10일 순차 치료에 비해서 유의하게 높았으나, 그 차이는 크지 않았다(RR 1.04; 95% CI 1.00-1.08 in ITT analysis; RR 1.04; 95% CI 1.01-1.07 in PP analysis) (Supplementary Fig. 5). 동시 치료의 제균율이 순차 치료에 비해서 근소하게 높았는데, 이는 clarithromycin이나 metronidazole 중 하나에만 내성이 있을 경우 동시 치료가 순차 치료에 비해 효과적이기 때문으로 생각된다. 실제로 clarithromycin에 내성이 있는 경우 동시 치료가 순차 치료에 비해 제균율이 높았으며[131,132], metronidazole에 내성이 있지만 clarithromycin에 내성이 없는 경우에서도 동시 치료가 순차 치료에 비해 제균율이 높았다[131,133].
6개 RCT에서 10일 동시 치료와 10일/14일 bismuth 4제요법의 제균율을 비교하였다(Supplementary Fig. 6). 10일 동시 치료의 제균율은 bismuth 4제요법의 제균율과 유의한 차이가 없었다(RR 1.05; 95% CI 0.96-1.15 in ITT analysis; RR 1.01; 95% CI 0.97-1.06 in PP analysis). 2개 RCT에서 10일 동시 치료와 병합 요법의 제균율을 비교하였는데, 양군의 제균율은 차이가 없었다(RR 0.99; 95% CI 0.93-1.05 in ITT analysis). 3개의 RCT에서 14일 동시 치료와 10일/14일 순차 치료의 제균율을 비교하였다. 14일 동시 치료의 제균율은 ITT 분석에서는 순차 치료와 차이가 없었으나(76% vs. 79%), PP 분석에서는 14일 동시 치료가 순차 치료에 비해서 제균율이 약간 높았다(89% vs. 82%). 2개의 RCT에서 14일 동시 치료와 14일 표준 3제요법의 제균율을 비교하였으며, 14일 동시 치료의 제균율이 14일 표준 3제요법의 제균율에 비해 유의하게 높았다(88% vs. 79% in ITT analysis; 94% vs. 82% PP analysis). 2개의 RCT에서는 14일 동시 치료와 병합 요법의 제균율을 비교하였는데, 14일 동시 치료의 제균율이 병합 요법의 제균율에 비해 약간 높았다(91% vs. 85%, p= 0.05 in ITT analysis; 96% vs. 92%, p= 0.07 in PP analysis).
요약하면, 1차 제균 치료로서 10일 동시 치료의 제균율이 10일/14일 표준 3제요법에 비해서는 유의하게 높았으나, 순차 치료에 비해서는 근소하게 높고, 병합 요법 및 bismuth 4제요법과는 유사하였다. 14일 동시 치료는 14일 표준 3제요법과 비교 시 PP 및 ITT 분석 모두에서 유의하게 제균율이 높았으며, 10일/14일 순차 치료에 비해서는 PP 분석에서 제균율이 높았다. 10일 동시 요법과 14일 동시 요법의 제균율은 비슷하였다. 따라서 내성 검사 없이 1차 치료를 고려하는 경우, 10일 동시 치료가 권장된다.
Clarithromycin 내성 검사는 표준 3제요법의 제균율을 향상시키는가?
지침 7. 7일 동안의 표준 3제요법을 1차 제균요법으로 사용하고자 할 때에는 중합효소연쇄반응 또는 염기서열법을 이용한 clarithromycin 내성 검사를 권고한다.
권고 강도: 강함
근거 수준: 낮음
전문가 합의: 83.3% (1차)
우리나라의 경험적 H. pylori 감염 표준 3제요법의 제균율은 점차 감소하여 약 70% 정도이다[134-136]. Maastricht V 가이드라인에서는 clarithromycin의 내성이 15%를 초과하는 지역에서는 경험적 표준 3제요법을 1차 치료로 사용하지 않을 것을 강력히 권고하고 있다[92]. 우리나라에서 표준 3제요법의 낮은 제균율과 clarithromycin에 대한 높은 내성률을 고려하면, H. pylori 감염의 치료에 대한 새로운 전략이 필요한 시점이다.
H. pylori를 배양하고 항생제 감수성 검사 결과를 토대로 항생제를 선택하는 것이 가장 이상적인 방법이지만[137,138], H. pylori는 배양 기간이 오래 걸리고, 배양 환경이 까다롭기 때문에 배양이 쉽지 않아 임상에 적용하기가 어렵다. 그러나, clarithromycin의 내성과 관련이 있다고 알려진 23S 리보솜 RNA의 점돌연변이를 찾아내는 중합효소연쇄반응(polymerase chain reaction, PCR) 또는 염기서열법을 이용하여 약제를 선택하는 방법은 임상에서 비교적 쉽게 사용할 수 있다.
국내 1,232명을 대상으로 시행된 환자-대조군 연구에서, dual priming oligonucleotide-based multiplex PCR법을 이용하여 A2142G, A2143G 부위에 점돌연변이를 가지고 있는 환자군은 PPI + amoxicillin + metronidazole (PAM)로 7일 동안 치료하고, 점돌연변이가 없는 환자군은 표준 3제요법 7일 동안 치료하는 맞춤 치료를 시행하였고, 대조군은 7일 동안의 경험적 표준 3제요법을 시행하여 H. pylori 제균율을 비교하였다. 맞춤 치료 환자군의 제균율은 ITT 분석에서 80.7% (176/218)로 경험적 치료를 시행한 대조군의 표준 3제요법(69.5% [214/308]; p= 0.004) 또는 PAM (71.1% [219/308]; p= 0.012)의 제균율보다 높았다[79]. 최근에 발표된 두 개의 환자-대조군 연구 결과를 살펴보면, bismuth 4제요법, PAM, 또는 표준 3제요법을 이용한 7일 동안의 맞춤형 치료의 PP 분석 제균율이 각각 91.8%와 94.3%로, 7일 동안의 경험적 표준 3제요법 대조군의 72.1%와 76.5%보다 높았다[17,18]. 특히, 맞춤 치료의 비용은 14일 동안의 경험적 표준 3제요법의 비용과 거의 동등하여 비용-효과 측면에서도 열등하지 않았다[18].
Clarithromycin 내성이 있는 환자의 제균요법은 현재까지 발표된 연구를 근거로 7일 동안의 PAM 또는 bismuth 4제요법을 고려할 수 있다[16,17,78]. 그러나 최근 국내 연구에서는 clarithromycin 내성이 있는 환자에서 7일 동안의 PAM 치료의 제균율이 만족스럽지 않아(55.4% [51/92] in ITT analysis, 66.2% [51/77] in PP analysis) metronidazole의 용량을 높이고 치료기간을 늘려야 할 필요성을 제시하였다[139]. 따라서 clarithromycin 내성이 있는 경우에서의 제균요법에 대한 권고안은 현재 우리나라에서 진행 중인 RCT 결과에 근거하여 추가해야 할 것이다. 요약하면, 7일 동안의 제균요법을 고려할 때에는 임상에 적용 가능한 검사법을 이용하여 clarithromycin 내성 검사를 시행하고, 내성이 없는 환자에게 표준 3제요법을 권고한다.
Bismuth 4제요법은 헬리코박터 파일로리 감염의 1차 제균치료로 사용될 수 있는가?
지침 8. Bismuth 4제요법 (양성자펌프억제제 표준 용량 하루 2회, metronidazole 500 mg 하루 3회, bismuth 120 mg과 tetracycline 500 mg 하루 4회 10일에서 14일간)은 14일 표준 3제요법, 10일 동시 치료 및 순차 치료와 유사한 1차 치료 제균율을 보이나 약물 부작용이 높고 구제요법으로 사용할 가능성이 있어 다른 제균 치료를 사용할 수 없는 경우에 1차 치료로 사용할 것을 권고한다.
권고 강도: 약함
근거 수준: 중간
전문가 합의: 83.3% (1차)
H. pylori의 항생제 내성은 제균율에 직접적인 영향을 주는데, 그중에서도 clarithromycin 내성이 H. pylori 제균 성공 여부에 가장 중요한 요인으로 알려져 있다. 여러 가이드라인들에 따르면, clarithromycin 내성률이 높은 지역에서는 1차 치료로 bismuth를 기본으로 한 4제요법 또는 bismuth를 포함하지 않은 4제요법을 사용할 것을 권고하고 있다[34,140]. 일반적으로 clarithromycin 내성이 높은 지역의 정의는 일정 지역에서 clarithromycin 내성이 있는 H. pylori가 15% 이상일 때를 뜻한다[34]. 우리나라의 경우, 지난 10년 동안 H. pylori의 clarithromycin 내성률은 점차적으로 증가해왔으며[11], 최근 논문들에서도 국내의 clarithromycin 내성률은 17.8-31.0%로 보고하였다[141,142].
Bismuth를 포함한 4제요법과 관련된 RCT들을 이용한 두 개의 네트워크 메타분석 연구 결과, bismuth를 기본으로 한 4제요법의 효과는 항생제의 조합과 치료 기간에 따라 상당한 차이가 있는 것으로 나타났다[143,144]. 이에, 본 지침에서는 2008년 1월부터 2018년 7월까지 1차 치료로 bismuth를 기본으로 한 4제요법을 사용한 9개의 RCT들을 대상으로 bismuth를 기본으로 한 4제요법의 효과와 안전성을 확인하기 위한 메타분석을 시행하였다(Supplementary Table 7) [108,115,120,145-150]. Bismuth를 기본으로 한 4제요법의 제균율은 ITT 분석에서는 84.5% (95% CI 74.9-90.9%), PP 분석에서는 90.6% (95% CI 82.8-95.1%)로 확인되었다. 그러나, 다른 제균 치료법들의 제균율과 비교한 ITT 분석 결과에서는 bismuth를 기본으로 한 4제요법 10-14일의 제균율이 14일 표준 3제요법(RR 1.28; 95% CI 0.97-1.70) (Fig. 7A), 10일 순차 치료(RR 0.96; 95% CI 0.83-1.12) (Fig. 7B), 10일 동시 치료(RR 1.01; 95% CI 0.93-1.10) (Fig. 7C)의 제균율에 비해 통계적으로 우월함을 보여주지 못 하였다.
또한, PP 분석에서도 bismuth를 기본으로 한 4제요법 10-14일의 제균율은 14일 표준 3제요법(RR 1.37; 95% CI 0.95-1.99), 10일 순차 치료(RR 0.99; 95% CI 0.93-1.05), 10일 동시 치료(RR 1.01; 95% CI 0.95-1.07)에 비해 통계적으로 유의한 차이가 없었다. 이번 메타분석에 포함된 논문들의 이질성은 전반적으로 중간 정도였으나, 결과 해석에 있어 분석에 이용된 연구의 수가 적다는 제한점은 감안해야 할 것이다.
부작용 측면에서는 연구들 사이에 상당한 이질성이 있는 것으로 확인되었으나(I2 = 92%), bismuth를 기본으로 한 4제요법의 부작용이 다른 제균 치료들에 비해 유의하게 높은 것으로 나타났다(RR 1.72; 95% CI 1.23-2.40) (Supplementary Fig. 7).
Bismuth를 기본으로 한 4제요법은 페니실린 알레르기가 있는 환자들이나 중복 내성이 있는 H. pylori에 감염된 환자들에서도 효과적으로 사용할 수 있는 제균 치료법이기 때문에[140], 임상의들에게 유용한 치료 방법 중 하나일 수 있다. 하지만, 실제 임상에서 bismuth를 기본으로 한 4제요법을 1차 치료법으로 사용하기에는 bismuth를 기본으로 한 4제요법의 높은 부작용과 1차 치료에 실패하였을 때 사용할 수 있는 구제요법이 적다는 현실에 직면하게 된다. 따라서, bismuth를 기본으로 한 4제요법은 다른 1차 치료법들을 사용할 수 없는 경우에 일차 치료로 사용할 것을 권고하며, 향후 1차 bismuth를 기본으로 한 4제요법의 효과를 확인하기 위한 잘 디자인된 국내 연구가 필요하겠다.
추가적으로, bismuth의 용량과 관련하여 국내에서 사용하는 bismuth subcitrate인 DeNol® 300 mg에는 elemental bismuth 120 mg이 들어있다. 따라서, 임상에서 bismuth를 사용할 때에는 bismuth subcitrate를 기준으로 하여 300 mg을 하루 네 번 사용하는 것이 권고된다.
헬리코박터 파일로리 제균 치료가 실패한 이후 추천되는 구제요법은 무엇인가?
체계적 문헌 검색에서 하나 이상의 구제요법을 비교한 RCT들은 총 36개 검색되었다[151-186]. 이들을 이전에 실패한 요법에 따라 분류한 결과는 Supplementary Table 8에 정리되어 있으며, 이들 연구들은 비교한 구제요법 혹은 이전에 실패한 요법이 서로 매우 달라서 메타분석이 가능한 경우가 많지 않았다. 메타분석은 3개 이상의 RCT가 있는 경우에 시행하였다. 아래 언급되는 모든 제균율은 ITT 제균율이며 근거들은 Supplementary Table 9에 요약되어 있다.
지침 9. 1차 제균 치료로서 표준 3제요법에 실패한 경우 2차 제균 치료로서 bismuth 포함 4제 14일 요법을 권고한다.
권고 강도: 강함
근거 수준: 높음
전문가 합의: 96.7% (1차)
Bismuth 4제요법은 9개의 RCT에서 제균율이 75.5% (95% CI 71.6-79.1%)였다(Fig. 8) [160,165,166,169,171,179,180,183,186]. 치료 기간은 7일, 10일 혹은 14일이었으며, 10일 치료 요법과 14일 치료 요법이 비슷한 효과를 보였기 때문에, 하위 분석에서 7일 치료 요법과 10-14일 치료 요법을 비교하였다. 그 결과, 10-14일 치료 요법의 제균율이 81.6% (95% CI 76.9-85.6%; I2=29.6%)로 7일 치료 요법의 제균율 68.4% (95% CI 53.0-73.5%; I2=73.8%)보다 유의하게 높았다(p<0.01). Bismuth 4제요법의 14일 치료를 7일 치료를 직접 비교한 3개의 RCT의 메타분석에서도 14일 치료가 7일 치료보다 유의하게 높은 제균율을 보였다(risk difference [RD] 0.09; 95% CI 0.02-0.15).
Levofloxacin 3제요법은 8개의 RCT에서 제균율이 73.1% (95% CI 68.4-77.3%)였다(Fig. 9) [151,156,169,170,172,179,180,186]. 하위 그룹 분석에서 10-14일 치료 요법의 제균율이 78.5% (95% CI 71.9-84.0%)로 7일 치료 요법의 69.1% (95% CI 61.6-74.9%)보다 유의하게 높았다(p= 0.04). Levofloxacin 3제요법의 10일 치료와 7일 치료를 직접 비교한 1개의 RCT에서도 10일 치료 요법이 7일 치료 요법보다 제균율이 유의하게 높았다(87.5% vs. 67.5%, p< 0.01) [181].
Bismuth 4제요법과 levofloxacin 3제요법을 직접 비교한 4개의 RCT의 메타분석에서 두 요법의 제균율에 유의한 차이는 없었다[169,179,180,186]. 다만, ITT 분석에서는 levofloxacin 3제요법이 우월한 경향을 보였고(bismuth 4제요법 vs. levofloxacin 3제요법; RD -0.06; 95% CI -0.14 to 0.02) (Supplementary Fig. 8), PP 분석에서는 bismuth 4제요법이 우월한 경향을 보였다(RD 0.02; 95% CI -0.05 to 0.10) (Supplementary Fig. 8). 이는 bismuth 4제요법의 복약 순응도가 낮기 때문일 것으로 생각된다. 2006년에 발표된 2개의 체계적 문헌 고찰에서 levofloxacin 3제요법 10일 치료가 bismuth 4제요법 7일 치료보다 우월한 제균 효과를 보였으나[187,188], 본 메타분석에 포함된 연구들에서는 두 치료법을 서로 같은 기간 동안 사용하였다는 차이가 있다(7일 vs. 7일, 10일 vs. 10일, 14일 vs. 14일).
Levofloxacin 3제요법의 주요 제한점은 levofloxacin 내성이 있는 경우 제균 효과가 상당히 감소한다는 것이다[169]. 최근 한국인의 H. pylori 항생제 내성 전국 조사에서 levofloxacin 내성률이 37.0%였다[60]. 그러므로, 우리나라에서는 levofloxacin 3제요법보다는 bismuth 4제요법이 바람직하다고 할 수 있다. Metronidazole의 내성률도 29.5%로 높지만 치료 용량과 기간을 증가시켜 극복할 수 있기 때문에[189], bismuth 4제요법을 구제요법으로 사용할 때는 14일 치료가 10-14일 치료보다 바람직하다. 그러므로 일차 제균 치료로서 표준 3제요법에 실패한 경우 이차 제균 치료로서 bismuth 4제요법 14일 치료를 권고한다.
지침 10. 1차 치료로서 순차 치료 혹은 동시 치료에 실패한 경우 2차 제균 치료로서 bismuth 4제요법을 권고한다.
권고 강도: 강함
근거 수준: 매우 낮음
전문가 합의: 90.0% (1차)
문헌 검색에서 1차 제균 치료로서 순차 치료 또는 동시 치료에 실패한 환자에서 2차 제균 치료법을 비교한 RCT는 없었다. 코호트 연구들의 메타분석에서 levofloxacin 3제요법은 5개 연구 86명의 환자에서 제균율이 81% (95% CI 71-91%; I2 = 28%)였다[190]. 또 다른 메타분석에서는 순차 치료 실패 후 제균율이 81% (6개 연구; 95% CI 73-90%; I2=19%), 동시 치료 실패 후 제균율은 78% (3개 연구; 95% CI 58-97%; I2 = 67%)였다[34]. 그러나 상기에서 언급한 바와 같이 이러한 결과를 levofloxacin 내성률이 높은 국내에서 직접 적용하기는 어렵다[11,60,191,192].
Bismuth 4제요법은 코호트 연구들의 메타분석에서 제균률이 84% (95% CI 63-100%; I2 = 56%)였다[34]. 그러나 이 분석에서는 두 개의 코호트 연구에서 약 30명의 환자만이 포함되었다.
그러므로, 1차 제균 치료로서 순차 치료 혹은 동시 치료에 실패한 경우 2차 제균 치료로서 bismuth 4제요법을 권고한다. 그러나 이러한 권고를 뒷받침하기 위한 자료가 좀 더 필요하다.
지침 11. 1차 혹은 2차 치료로서 bismuth 4제요법에 실패한 경우 levofloxacin 포함 3제요법을 고려할 수 있다.
권고 강도: 약함
근거 수준: 매우 낮음
전문가 합의: 70.0% (1차)
1차 제균 치료로서 표준 3제요법, 순차 치료, 혹은 동시 치료에 실패한 뒤에 2차 치료로서 bismuth 4제요법에 실패하였다면, 3차 제균 치료 요법에 clarithromycin을 다시 사용하는 것은 추천되지 않는다. 1차 제균 치료로서 bismuth 4제요법에 실패한 경우에도 clarithromycin을 사용하는 것은 부적절할 수 있다. Bismuth 4제요법을 1차 제균 치료로 선택한 이유가 clarithromycin 내성이 의심되는 상황이었을 가능성이 많기 때문이다[193]. 배양 검사, PCR 혹은 염기서열법을 이용한 항생제 감수성 검사를 기반으로 치료법을 결정할 수도 있다. 일반적으로 clarithromycin, fluoroquinolone, rifabutin에 대한 내성이 있는 경우에는 해당 항생제를 다시 사용하는 것이 추천되지 않는다. 한편, amoxicillin과 metronidazole은 다시 사용할 수 있다[189]. 그러나, 1차 치료와 달리, 2차 혹은 3차 치료에서 이전에 실패한 항생제를 알고 있다면, 이를 바탕으로 한 경험 치료도 항생제 감수성 검사를 기반으로 한 치료법과 효과가 비슷할 수 있다[138]. 문헌 검색에서 1차 혹은 2차 제균 치료로서 bismuth 4제요법이 실패한 뒤에 구제요법을 비교한 RCT는 없었다. 따라서, 코호트 연구 결과 혹은 다른 상황에 사용된 RCT 결과를 바탕으로 다음과 같은 제균요법들을 고려할 수 있다. 이상의 내용을 종합한 H. pylori 치료의 1차 및 2차 치료법들과 알고리즘은 각각 Table 4 및 Fig. 10에 정리되어 있다.
코호트 연구들의 메타분석에서 levofloxacin 3제요법은 5개 연구 501명에서 제균율이 70.0% (95% CI 62.4-76.6%; I2 = 58.5%)였으며[34], 2017년 우리나라 14개 기관의 후향적 코호트 연구에서 levofloxacin 3제요법의 제균율은 56.9% (62/109)였다[194]. 이렇게 낮은 제균율의 원인은 우리나라의 높은 levofloxacin 내성률 때문으로 추정할 수 있다[11,60,191,192]. 그러므로, 1차 혹은 2차 제균 치료로서 bismuth 4제요법에 실패한 경우 levofloxacin 3제요법(PPI표준 용량 하루 2회, amoxicillin 1 g 하루 2회, levofloxacin 500 mg 하루 1회 혹은 250 mg 하루 2회) 10일 치료를 권고한다. 그러나, 이 요법의 제균율은 외국 연구 결과보다 낮을 수 있다는 점을 고려해야 한다.
일본 및 유럽에서 sitafloxacin, gatifloxacin, 혹은 moxifloxacin을 이용한 3제요법의 구제 치료 효과를 보고한 바 있으나[154,164,185], bismuth 4제요법에 실패한 뒤에 사용한 결과는 없었다. 우리나라 후향적 코호트 연구에서 1차 제균 치료로서 bismuth 4제요법에 실패한 뒤에 사용된 moxifloxacin 3제요법 7-14일 치료의 제균율은 67.9% (95% CI 51.5-84.9%)에 불과하였다[195]. 그러므로, bismuth 4제요법에 실패한 뒤 기타 fluoroquinolone 3제요법에 대한 근거는 제한적이다. Fluoroquinolone 사이의 잠재적인 교차 내성이 있기 때문에 levofloxacin의 대안으로 기타 fluoroquinolone을 사용하는 것은 더욱 제한된다고 할 수 있다[192].
Fluoroquinolone 4제요법은 bismuth 4제 요법이 아닌 다른 요법에 실패한 뒤에 사용된 RCT 결과만 보고되고 있어, 아직은 참고만 할 수 있는 상황이다[151,155,156,161,166,167,173,175].
Levofloxacin-bismuth 4제요법(levofloxacin, bismuth, PPI, amoxicillin)은 bismuth와 levofloxacin의 상승 효과로 항생제 내성을 극복할 수 있다는 근거로 Maastricht V 가이드라인에서 bismuth 4제요법 실패 후에 사용할 수 있는 “고무적인 구제요법”으로 제안되었다[34]. 두 개의 코호트 연구에서 2차 제균 치료로서 bismuth 4제요법에 실패한 뒤에 제균율이 83.8% (31/37; 95% CI 71.3-96.2%)이었다[196]. 다른 상황에서 구제요법으로 사용된 경우 제균율은 73-90%로 상이한 결과가 보고되었다[151,161,197,198]. 그러므로, 비록 이 요법이 효과적일 것으로 기대되나, 한국인에서 사용되기 전에 검증될 필요가 있다.
H. pylori 제균 치료에서 rifabutin을 이용하는 주요 제한점은 높은 가격, 골수 독성, 및 Mycobacterium tuberculosis에 대한 내성 유발에 관한 염려이다[199]. 2012년에 보고된 체계적 문헌 고찰에서 rifabutin 3제요법의 2차, 3차, 및 4차 제균 치료 요법으로서 제균율이 각각 79% (95% CI 67-92%), 66% (95% CI 55-77%), 및 70% (95% CI 60-79%)였다[199]. 포함된 논문들에서 사용한 rifabutin의 용량은 150 mg bid 혹은 300 mg qd였고 PPI와 amoxicillin과 함께 7일에서 10일간 사용하였다. 그러므로, rifabutin 3제요법은 bismuth 4제요법을 포함하여 여러 차례 제균에 실패한 뒤에 사용할 수 있는 구제요법의 하나로 제안될 수 있다. 그럼에도 불구하고, 상대적으로 부족한 근거와 알려진 위험을 신중하게 고려할 필요가 있다.
고용량 2제요법은 amoxicillin의 혈중 최저 농도를 높게 유지하기 위하여 하루 3 g 이상, 하루 3회 이상 투약하는 것으로 정의된다[140]. 하나의 RCT에서 1차례 이상 특정되지 않은 요법에 실패한 환자들을 대상으로 rabeprazole 20 mg과 amoxicillin 750 mg 하루 4회 14일 요법의 제균율이 89.3% (50/56; 95% CI 80.9-97.6%)였다[158]. 2008년 이전에 보고된 두 개의 RCT에서는 제균율은 각각 70-76%였다[200,201]. 이 요법 역시 bismuth 4제요법 실패 후 구제요법으로 고려될 수 있으나, 이를 뒷받침할 수 있는 자료가 더 필요하다.
비록 clarithromycin 포함 요법에 실패한 뒤에 clarithromycin을 다시 사용하는 것은 부적절하지만, 동시 치료는 clarithromycin과 metronidazole의 병용요법이 clarithromycin 내성을 극복할 수도 있다는 측면에서 bismuth 4제요법 실패 후 구제요법으로 선택될 수 있다[189]. 그러나 근거 자료는 매우 부족한 상황이다. 한 RCT에서 표준 3제요법에 실패한 뒤에 동시 치료 7일 요법이 86.5% (45/51; 95% CI 76.9-96.1%)의 제균율을 보였다[178]. 다른 RCT 내부에서 시행된 전향적 코호트 연구에서는 bismuth 4제요법에 실패한 뒤에 동시 치료 10일 요법이 84.6% (11/13; 95% CI 57.8-95.7%)의 제균율을 보였다[202].
H. pylori는 국내 유병률이 50% 내외에 달하는 매우 흔한 감염이며 소화성 궤양 및 위암 등의 질환을 일으키는 균으로 이에 대한 치료 적응증을 확립하고 효과적인 1차 및 2차 제균요법을 제시하는 것은 임상적으로 매우 중요하며 국가적으로 의료 비용을 효율적으로 사용하는 데 필요하다. 근래에 H. pylori의 clarithromycin에 대한 내성률이 증가함에 따라 기존의 표준 3제요법의 제균율은 하락하는 추세이며, 이를 극복하기 위한 방안으로 치료 기간을 늘리거나 순차 치료나 동시 치료와 같은 bismuth를 포함하지 않는 4제요법, clarithromycin 내성 검사를 이용하는 방법 그리고 bismuth 4제요법과 같은 방안이 이용될 수 있다. 구제요법의 경우 다양한 1차 제균요법이 사용됨에 각 요법에 따른 전략이 필요한데, 구제요법에 대한 RCT 논문들의 메타분석 결과, 표준 3제요법, 순차 치료, 동시 치료에서 실패한 경우 bismuth 4제요법이 권장된다. 일차 치료로 bismuth 4제요법을 사용하였을 때, 구제 치료로는 levofloxacin 3제요법이 권장된다.
이 지침은 최신 문헌들에 대한 메타분석과 동시에 근거 기반의 가이드라인 제작 방법을 사용하였으며 권고문은 한국의 내성률을 고려하여 만들어 졌으며, 따라서 한국과 항생제 내성률이 유사한 극동 지역에서 적용이 가능할 것으로 생각된다. 그리고 최근에 도입된 PCR과 염기서열법에 기반한 clarithromycin 내성 검사 기법을 이용한 제균 치료에 대한 최신의 문헌들을 기반으로 한 권고문도 작성되어 이의 사용을 고려하는 임상 상황에서 도움을 줄 것으로 생각한다.
이번 지침에서 만성 위축성 위염 및 장상피화생에 대한 제균 치료의 적응증에 대한 권고문은 전문가들의 합의에 도달하지 못하였다. 이 적응증은 매우 중요한 주제로 향후 만성 위축성 위염 및 장상피화생이 있는 환자에서 H. pylori 제균 치료가 위암의 발생을 낮출 수 있는지에 대한 장기적인 잘 계획된 연구가 필요해 보인다. 또한 위암의 가족력 역시 위암의 위험 인자로 잘 알려져 있으며 이 환자군에 대한 치료 적응증을 확립하기 위한 추가 연구가 필요할 것으로 생각된다. 또한 일차 요법 간의 비용 효율성에 대한 연구가 이뤄진다면 이번 가이드라인에 제시된 다양한 1차 치료 요법들 중 어떤 환자나 임상 상황에서 어떤 치료를 선택하는 게 가장 효율적인지에 대한 결정에 도움을 줄 수 있을 것으로 보인다.
Supplementary Materials
Supplementary Table 1.
Studies included in meta-analysis about the efficacy of H. pylori eradication after endoscopic resection of
gastric adenoma
Supplementary Table 2.
Studies included in meta-analysis about the efficacy of H. pylori eradication in patients with functional dyspepsia
Supplementary Table 3.
Evidence table about the eradication rate of standard triple therapy in H. pylori infection
Supplementary Table 4.
Evidence table about the eradication rate of ST in H. pylori infection
Supplementary Table 5.
Studies included in meta-analysis about the eradication rate of CT in H. pylori eradication
Supplementary Table 6.
Pooled eradication rate of concomitant therapy from randomized controlled trials with random effect model
Supplementary Table 8.
Categorization of salvage regimens from overall 36 randomized controlled trials with 77 intervention arms
Supplementary Table 9.
Evidence table of 16 RCTs which were included in the meta-analysis of regimens for salvage therapy
Supplementary Figure 1.
Meta-analysis of efficacy of Helicobacter pylori eradication in patients with functional dyspepsia. M-H, Mantel-Haenszel test; ORCHID, Optimal Regimen Cures Helicobacter Induced Dyspepsia Study Group.
Supplementary Figure 2.
Meta-analysis of effect of Helicobacter pylori eradication on gastric cancer development. (A) Forest plot of studies reporting gastric cancer in eradication group and control group in subjects with no precancerous lesions at baseline. (B) Forest plot of studies reporting gastric cancer in eradication group and control group in subjects with atrophic gastritis, intestinal metaplasia, and dysplasia at baseline. M-H, Mantel-Haenszel test.
Supplementary Figure 3.
Pooled eradication rates of standard triple therapy by treatment duration from randomized controlled trials performed in Korea. Eradication rates of standard triple therapy (STT) for 7 days were 70.0% (95% CI 68.5-71.4%) in intention-to-treat (ITT) analysis and 78.4% (95% CI 77.0-79.7%) in per protocol (PP) analysis. Eradication rates of STT for 10 days were 73.7% (95% CI 69.8-77.2%) in ITT analysis and 78.9% (95% CI 74.9-82.4%) in PP analysis. Eradication rates of STT for 14 days were 78.1% (95% CI 75.2-80.7%) in ITT analysis and 86.0% (95% CI 83.4-88.3%) in PP analysis. ap < 0.05.
Supplementary Figure 4.
Forest plot comparing the different therapies in terms of the eradication rates between sequential therapy. Intention-to-treat analysis. BQT, bismuth quadruple therapy; M-H, Mantel-Haenszel test.
Supplementary Figure 5.
Comparison between 10-day concomitant therapy and 10-day sequential therapy. (A) Intention-to-treat analysis. (B) Per protocol analysis. CT, concomitant therapy; SQT, sequential therapy; M-H, Mantel-Haenszel test.
Supplementary Figure 6.
Comparison between 10-day concomitant therapy and 10-/14-day bismuth quadruple therapy. (A) Intention-to-treat analysis. (B) Per protocol analysis. CT, concomitant therapy; BQT, bismuth quadruple therapy; M-H, Mantel-Haenszel test.
Supplementary Figure 7.
Comparison of adverse events between 10-/14-day bismuth quadruple therapy and other regimens (standard triple therapy, sequential therapy, concomitant therapy). PBMT, bismuth quadruple therapy; M-H, Mantel-Haenszel test.
Supplementary Figure 8.
Meta-analysis of four studies which compared bismuth quadruple therapy and levofloxacin triple therapy after failure of first-line standard triple therapy. (A) Intention-to-treat analysis. (B) Per protocol analysis. PBTM, bismuth quadruple therapy; PAL, proton pump inhibitor, amoxicillin, levofloxacin; M-H, Mantel-Haenszel test.
Acknowledgements
We would like to express our deep gratitude to Miyoung Choi, PhD of National Evidence based Healthcare Collabo- rating Agency who performed initial literature search for systematic review, Ein Soon Shin, PhD & MPH, Research Head of the Research Agency for Clinical Practice Guidelines of Korean Academy of Medical Sciences for the Meta-Analysis Workshop, and Soo Young Kim, a professor from the Department of Family Medicine, Hallym University College of Medicine for conducting the Workshop on Expert Consensus Method. We also thank Nayoung Kim, a professor from the Department of Internal Medicine, Seoul National University College of Medicine and Gwang Ha Kim, a professor from the Department of Internal Medicine, Pusan University College of Medicine who reviewed the draft of the guidelines by peer review. Finally, we thank the Internet community 'Bokanyi' for helping us with the patient preference survey.
REFERENCES
1. Hunt RH, Xiao SD, Megraud F, et al. Helicobacter pylori in developing countries. World Gastroenterology Organisation Global Guideline. J Gastrointestin Liver Dis 2011;20:299–304.

2. Lee JH, Ahn JY, Choi KD, et al. Nationwide antibiotic resistance mapping of Helicobacter pylori in Korea: a prospective multicenter study. Helicobacter 2019;24:e12592.


3. Statistics Korea. Vital Statistics of Korea PeriodAnnual 1970-2018 [Internet]. Daejeon: Statistics Korea, 2019. [cited 2017 Dec 31]. Available from: http://kostat.go.kr/wnsearch/search.jsp
4. Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015;64:1353–1367.



5. Yeh JM, Kuntz KM, Ezzati M, Hur C, Kong CY, Goldie SJ. Development of an empirically calibrated model of gastric cancer in two high-risk countries. Cancer Epidemiol Biomarkers Prev 2008;17:1179–1187.


6. Calam J, Gibbons A, Healey ZV, Bliss P, Arebi N. How does Helicobacter pylori cause mucosal damage? Its effect on acid and gastrin physiology. Gastroenterology 1997;113(6 Suppl):S43–S50.


7. De Francesco V, Giorgio F, Hassan C, et al. Worldwide H. pylori antibiotic resistance: a systematic review. J Gastrointestin Liver Dis 2010;19:409–414.

8. Liou JM, Chen PY, Kuo YT; Wu MS; Taiwan Gastrointestinal Disease and Helicobacter Consortium. Toward population specific and personalized treatment of Helicobacter pylori infection. J Biomed Sci 2018;25:70.



9. Kim JM, Kim JS, Jung HC, Kim N, Kim YJ, Song IS. Distribution of antibiotic MICs for Helicobacter pylori strains over a 16-year period in patients from Seoul, South Korea. Antimicrob Agents Chemother 2004;48:4843–4847.



10. Hwang TJ, Kim N, Kim HB, et al. Change in antibiotic resistance of Helicobacter pylori strains and the effect of A2143G point mutation of 23S rRNA on the eradication of H. pylori in a single center of Korea. J Clin Gastroenterol 2010;44:536–543.


11. Lee JW, Kim N, Kim JM, et al. Prevalence of primary and secondary antimicrobial resistance of Helicobacter pylori in Korea from 2003 through 2012. Helicobacter 2013;18:206–214.


12. Lee JY, Kim N, Nam RH, Choi SI, Lee JW, Lee DH. Primary and secondary antibiotic resistance of Helicobacter pylori in Korea from 2003 to 2018. Helicobacter 2019;24:e12660.


13. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928.



14. Kim SY, Park JE, Lee YJ, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol 2013;66:408–414.


15. Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE Handbook. Hamilton: GRADE Working Group, 2013.
16. Andrews J, Guyatt G, Oxman AD, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol 2013;66:719–725.


17. Cho JH, Jeon SR, Kim HG, Jin SY, Park S. Cost-effectiveness of a tailored Helicobacter pylori eradication strategy based on the presence of a 23S ribosomal RNA point mutation that causes clarithromycin resistance in Korean patients. J Gastroenterol Hepatol 2019;34:700–706.


18. Gweon TG, Kim JS, Kim BW. An economic modeling study of Helicobacter pylori eradication: comparison of dual priming oligonucleotide-based multiplex polymerase chain reaction and empirical treatment. Gut Liver 2018;12:648–654.



19. McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993-2005. Public Health Nutr 2009;12:444–454.


20. Stevens GA, Finucane MM, De-Regil LM, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: a systematic analysis of population-representative data. Lancet Glob Health 2013;1:e16–e25.



21. Zhang ZW, Patchett SE, Perrett D, Katelaris PH, Domizio P, Farthing MJ. The relation between gastric vitamin C concentrations, mucosal histology, and CagA seropositivity in the human stomach. Gut 1998;43:322–326.



22. Hudak L, Jaraisy A, Haj S, Muhsen K. An updated systematic re view and meta-analysis on the association between Helicobacter pylori infection and iron deficiency anemia. Helicobacter 2017;22:e12330.

23. Chen LH, Luo HS. Effects of H pylori therapy on erythrocytic and iron parameters in iron deficiency anemia patients with H pylori-positive chronic gastristis. World J Gastroenterol 2007;13:5380–5383.



24. Miernyk K, Bruden D, Zanis C, et al. The effect of Helicobacter pylori infection on iron stores and iron deficiency in urban Alaska native adults. Helicobacter 2013;18:222–228.



25. Monzón H, Forné M, Esteve M, et al. Helicobacter pylori infection as a cause of iron deficiency anaemia of unknown origin. World J Gastroenterol 2013;19:4166–4171.



26. Choi IJ, Kook MC, Kim YI, et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med 2018;378:1085–1095.


27. Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet 2008;372:392–397.


28. Choi JM, Kim SG, Choi J, et al. Effects of Helicobacter pylori eradication for metachronous gastric cancer prevention: a randomized controlled trial. Gastrointest Endosc 2018;88:475–485.e2.


29. Song JH, Yang SY, Lim JH, Choi JM, Kim SG. The effect of Helicobacter pylori eradication on the metachronous neoplasm after endoscopic resection for gastric dysplasia. Korean J Gastroenterol 2017;70:27–32.


30. Shin SH, Jung DH, Kim JH, et al. Helicobacter pylori eradication prevents metachronous gastric neoplasms after endoscopic resection of gastric dysplasia. PLoS One 2015;10:e0143257.



31. Chon I, Choi C, Shin CM, Park YS, Kim N, Lee DH. Effect of Helicobacter pylori eradication on subsequent dysplasia development after endoscopic resection of gastric dysplasia. Korean J Gastroenterol 2013;61:307–312.


32. Du LJ, Chen BR, Kim JJ, Kim S, Shen JH, Dai N. Helicobacter pylori eradication therapy for functional dyspepsia: systematic review and meta-analysis. World J Gastroenterol 2016;22:3486–3495.



33. Zhao B, Zhao J, Cheng WF, et al. Efficacy of Helicobacter pylori eradication therapy on functional dyspepsia: a meta-analysis of randomized controlled studies with 12-month follow-up. J Clin Gastroenterol 2014;48:241–247.


34. Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut 2017;66:6–30.


35. Moayyedi P, Lacy BE, Andrews CN, Enns RA, Howden CW, Vakil N. ACG and CAG clinical guideline: management of dyspepsia. Am J Gastroenterol 2017;112:988–1013.


36. McColl K, Murray L, El-Omar E, et al. Symptomatic benefit from eradicating Helicobacter pylori infection in patients with nonulcer dyspepsia. N Engl J Med 1998;339:1869–1874.


37. Blum AL, Talley NJ, O'Moráin C, et al. Lack of effect of treating Helicobacter pylori infection in patients with nonulcer dyspepsia. Omeprazole plus clarithromycin and amoxicillin effect one year after treatment (OCAY) study group. N Engl J Med 1998;339:1875–1881.


38. Talley NJ, Vakil N, Ballard ED 2nd, Fennerty MB. Absence of benefit of eradicating Helicobacter pylori in patients with nonulcer dyspepsia. N Engl J Med 1999;341:1106–1111.


39. Talley NJ, Janssens J, Lauritsen K, Rácz I, Bolling-Sternevald E. Eradication of Helicobacter pylori in functional dyspepsia: randomised double blind placebo controlled trial with 12 months' follow up. The optimal regimen cures helicobacter induced dyspepsia (ORCHID) study group. BMJ 1999;318:833–837.



40. Koskenpato J, Farkkilä M, Sipponen P. Helicobacter pylori eradication and standardized 3-month omeprazole therapy in functional dyspepsia. Am J Gastroenterol 2001;96:2866–2872.


41. Hsu PI, Lai KH, Tseng HH, et al. Eradication of Helicobacter pylori prevents ulcer development in patients with ulcer-like functional dyspepsia. Aliment Pharmacol Ther 2001;15:195–201.


42. Bruley Des Varannes S, Fléjou JF, Colin R, Zaïm M, Meunier A, Bidaut-Mazel C. There are some benefits for eradicating Helicobacter pylori in patients with non-ulcer dyspepsia. Aliment Pharmacol Ther 2001;15:1177–1185.


43. Froehlich F, Gonvers JJ, Wietlisbach V, et al. Helicobacter pylori eradication treatment does not benefit patients with nonulcer dyspepsia. Am J Gastroenterol 2001;96:2329–2336.


44. Chiba N, Van Zanten SJ, Sinclair P, Ferguson RA, Escobedo S, Grace E. Treating Helicobacter pylori infection in primary care patients with uninvestigated dyspepsia: the Canadian adult dyspepsia empiric treatment-Helicobacter pylori positive (CADET-Hp) randomised controlled trial. BMJ 2002;324:1012–1016.



45. Veldhuyzen van Zanten S, Fedorak RN, Lambert J, Cohen L, Vanjaka A. Absence of symptomatic benefit of lansoprazole, clarithromycin, and amoxicillin triple therapy in eradication of Helicobacter pylori positive, functional (nonulcer) dyspepsia. Am J Gastroenterol 2003;98:1963–1969.


46. Malfertheiner P, Mossner J, Fischbach W, et al. Helicobacter pylori eradication is beneficial in the treatment of functional dyspepsia. Aliment Pharmacol Ther 2003;18:615–625.


47. Gisbert JP, Cruzado AI, Garcia-Gravalos R, Pajares JM. Lack of benefit of treating Helicobacter pylori infection in patients with functional dyspepsia. Randomized one-year follow-up study. Hepatogastroenterology 2004;51:303–308.

48. Mazzoleni LE, Sander GB, Ott EA, et al. Clinical outcomes of eradication of Helicobacter pylori in nonulcer dyspepsia in a population with a high prevalence of infection: results of a 12-month randomized, double blind, placebo-controlled study. Dig Dis Sci 2006;51:89–98.


49. Ang TL, Fock KM, Teo EK, et al. Helicobacter pylori eradication versus prokinetics in the treatment of functional dyspepsia: a randomized, double-blind study. J Gastroenterol 2006;41:647–653.


50. Gwee KA, Teng L, Wong RK, Ho KY, Sutedja DS, Yeoh KG. The response of Asian patients with functional dyspepsia to eradication of Helicobacter pylori infection. Eur J Gastroenterol Hepatol 2009;21:417–424.

51. Mazzoleni LE, Sander GB, Francesconi CF, et al. Helicobacter pylori eradication in functional dyspepsia: HEROES trial. Arch Intern Med 2011;171:1929–1936.


52. Sodhi JS, Javid G, Zargar SA, et al. Prevalence of Helicobacter pylori infection and the effect of its eradication on symptoms of functional dyspepsia in Kashmir, India. J Gastroenterol Hepatol 2013;28:808–813.


53. Yazdanbod A, Salimian S, Habibzadeh S, Hooshyar A, Maleki N, Norouzvand M. Effect of Helicobacter pylori eradication in Iranian patients with functional dyspepsia: a prospective, randomized, placebo-controlled trial. Arch Med Sci 2015;11:964–969.


54. Kang SJ, Park B, Shin CM. Helicobacter pylori eradication therapy for functional dyspepsia: a meta-analysis by region and H. pylori prevalence. J Clin Med 2019;8:1324.


55. Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 2017;153:420–429.


56. Chen HN, Wang Z, Li X, Zhou ZG. Helicobacter pylori eradication cannot reduce the risk of gastric cancer in patients with intestinal metaplasia and dysplasia: evidence from a meta-analysis. Gastric Cancer 2016;19:166–175.


57. Rokkas T, Rokka A, Portincasa P. A systematic review and meta-analysis of the role of Helicobacter pylori eradication in preventing gastric cancer. Ann Gastroenterol 2017;30:414–423.



58. Yanaoka K, Oka M, Ohata H, et al. Eradication of Helicobacter pylori prevents cancer development in subjects with mild gastric atrophy identified by serum pepsinogen levels. Int J Cancer 2009;125:2697–2703.


59. Li WQ, Ma JL, Zhang L, et al. Effects of Helicobacter pylori treatment on gastric cancer incidence and mortality in subgroups. J Natl Cancer Inst 2014;106:dju116.



60. Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter 2007;12:275–278.


61. Choi HS, Park DI, Hwang SJ, et al. Double-dose, new-generation proton pump inhibitors do not improve Helicobacter pylori eradication rate. Helicobacter 2007;12:638–642.


62. Kim BG, Lee DH, Ye BD, et al. Comparison of 7-day and 14-day proton pump inhibitor-containing triple therapy for Helicobacter pylori eradication: neither treatment duration provides acceptable eradication rate in Korea. Helicobacter 2007;12:31–35.

63. Kim SY, Lee SW, Jung SW, et al. Comparative study of Helicobacter pylori eradication rates of twice-versus fourtimes-daily amoxicillin administered with proton pump inhibitor and clarithromycin: a randomized study. Helicobacter 2008;13:282–287.


64. Kim N, Park SH, Seo GS, et al. Lafutidine versus lansoprazole in combination with clarithromycin and amoxicillin for one versus two weeks for Helicobacter pylori eradication in Korea. Helicobacter 2008;13:542–549.


65. Choi WH, Park DI, Oh SJ, et al. Effectiveness of 10 day-sequential therapy for Helicobacter pylori eradication in Korea. Korean J Gastroenterol 2008;51:280–284.

66. Kim MN, Kim N, Lee SH, et al. The effects of probiotics on PPI-triple therapy for Helicobacter pylori eradication. Helicobacter 2008;13:261–268.


67. Jung JM, Shim KN, Oh HJ, et al. Role of anti-secretory treatment in addition to Helicobacter pylori eradication triple therapy in the treatment of peptic ulcer. Korean J Gastroenterol 2008;51:11–18.

68. Kim HW, Kim GH, Cheong JY, et al. H pylori eradication: a randomized prospective study of triple therapy with or without ecabet sodium. World J Gastroenterol 2008;14:908–912.



69. Lee JH, Jung HY, Choi KD, Song HJ, Lee GH, Kim JH. The Influence of CYP2C19 polymorphism on eradication of Helicobacter pylori : a prospective randomized study of lansoprazole and rabeprazole. Gut Liver 2010;4:201–206.



70. Song MJ, Park DI, Park JH, et al. The effect of probiotics and mucoprotective agents on PPI-based triple therapy for eradication of Helicobacter pylori. Helicobacter 2010;15:206–213.


71. Kim YS, Kim SJ, Yoon JH, et al. Randomised clinical trial: the efficacy of a 10-day sequential therapy vs. a 14-day standard proton pump inhibitor-based triple therapy for Helicobacter pylori in Korea. Aliment Pharmacol Ther 2011;34:1098–1105.


72. Choi KH, Chung WC, Lee KM, et al. Efficacy of levofloxacin and rifaximin based quadruple therapy in Helicobacter pylori associated gastroduodenal disease: a double-blind, randomized controlled trial. J Korean Med Sci 2011;26:785–790.



73. Chung JW, Jung YK, Kim YJ, et al. Ten-day sequential versus triple therapy for Helicobacter pylori eradication: a prospective, open-label, randomized trial. J Gastroenterol Hepatol 2012;27:1675–1680.


74. Choi HS, Chun HJ, Park SH, et al. Comparison of sequential and 7-, 10-, 14-d triple therapy for Helicobacter pylori infection. World J Gastroenterol 2012;18:2377–2382.



75. Park HG, Jung MK, Jung JT, et al. Randomised clinical trial: a comparative study of 10-day sequential therapy with 7-day standard triple therapy for Helicobacter pylori infection in na- ïve patients. Aliment Pharmacol Ther 2012;35:56–65.


76. Oh HS, Lee DH, Seo JY, et al. Ten-day sequential therapy is more effective than proton pump inhibitor-based therapy in Korea: a prospective, randomized study. J Gastroenterol Hepatol 2012;27:504–509.

77. Kim SY, Jung SW, Kim JH, et al. Effectiveness of three times daily lansoprazole/amoxicillin dual therapy for Helicobacter pylori infection in Korea. Br J Clin Pharmacol 2012;73:140–143.



78. Lee HJ, Kim JI, Cheung DY, et al. Eradication of Helicobacter pylori according to 23S ribosomal RNA point mutations associated with clarithromycin resistance. J Infect Dis 2013;208:1123–1130.


79. Kim SY, Lee SW, Hyun JJ, et al. Comparative study of Helicobacter pylori eradication rates with 5-day quadruple "concomitant" therapy and 7-day standard triple therapy. J Clin Gastroenterol 2013;47:21–24.


80. Heo J, Jeon SW, Jung JT, et al. A randomised clinical trial of 10-day concomitant therapy and standard triple therapy for Helicobacter pylori eradication. Dig Liver Dis 2014;46:980–984.


81. Park CS, Lee SM, Park CH, et al. Pretreatment antimicrobial susceptibility-guided vs. clarithromycin-based triple therapy for Helicobacter pylori eradication in a region with high rates of multiple drug resistance. Am J Gastroenterol 2014;109:1595–1602.


82. Lee JW, Kim N, Kim JM, et al. A comparison between 15-day sequential, 10-day sequential and proton pump inhibitor-based triple therapy for Helicobacter pylori infection in Korea. Scand J Gastroenterol 2014;49:917–924.


83. Lee HJ, Kim JI, Lee JS, et al. Concomitant therapy achieved the best eradication rate for Helicobacter pylori among various treatment strategies. World J Gastroenterol 2015;21:351–359.



84. Bang CS, Kim YS, Park SH, et al. Additive effect of pronase on the eradication rate of first-line therapy for Helicobacter pylori infection. Gut Liver 2015;9:340–345.


85. Chung JW, Han JP, Kim KO, et al. Ten-day empirical sequential or concomitant therapy is more effective than triple therapy for Helicobacter pylori eradication: a multicenter, prospective study. Dig Liver Dis 2016;48:888–892.

86. Kim JS, Kim BW, Hong SJ, et al. Sequential therapy versus triple therapy for the first line treatment of Helicobacter pylori in Korea: a nationwide randomized trial. Gut Liver 2016;10:556–561.



87. Kim BJ, Lee H, Lee YC, et al. Ten-day concomitant, 10-day sequential, and 7-day triple therapy as first-line treatment for Helicobacter pylori infection: a nationwide randomized trial in Korea. Gut Liver 2019;13:531–540.



88. Jung YS, Park CH, Park JH, et al. Efficacy of Helicobacter pylori eradication therapies in Korea: a systematic review and network meta-analysis. Helicobacter 2017;22:e12389.

89. Liu WZ, Xie Y, Lu H, et al. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter 2018;23:e12475.


90. Gao XZ, Qiao XL, Song WC, Wang XF, Liu F. Standard triple, bismuth pectin quadruple and sequential therapies for Helicobacter pylori eradication. World J Gastroenterol 2010;16:4357–4362.



91. Lahbabi M, Alaoui S, El Rhazi K, et al. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: result of the HPFEZ randomised study. Clin Res Hepatol Gastroenterol 2013;37:416–421.


92. Rakici H, Akdoğan RA, Bedir R, Copur A, Yilmaz A. Comparison of standard triple therapy, sequential therapy and moxifloxacin-based triple therapy for Helicobacter pylori infection: patients' compliance and bacterial eradication rates. J Dig Dis 2014;15:508–513.


93. Molina-Infante J, Perez-Gallardo B, Fernandez-Bermejo M, et al. Clinical trial: clarithromycin vs. levofloxacin in first-line triple and sequential regimens for Helicobacter pylori eradication. Aliment Pharmacol Ther 2010;31:1077–1084.


94. Javid G, Zargar SA, Bhat K, et al. Efficacy and safety of sequential therapy versus standard triple therapy in Helicobacter pylori eradication in Kashmir India: a randomized comparative trial. Indian J Gastroenterol 2013;32:190–194.


95. Morse AL, Goodman KJ, Munday R, et al. A randomized controlled trial comparing sequential with triple therapy for Helicobacter pylori in an Aboriginal community in the Canadian North. Can J Gastroenterol 2013;27:701–706.



96. Zhou L, Zhang J, Chen M, et al. A comparative study of sequential therapy and standard triple therapy for Helicobacter pylori infection: a randomized multicenter trial. Am J Gastroenterol 2014;109:535–541.


97. Lee H, Hong SN, Min BH, et al. Comparison of efficacy and safety of levofloxacin-containing versus standard sequential therapy in eradication of Helicobacter pylori infection in Korea. Dig Liver Dis 2015;47:114–118.


98. Greenberg ER, Anderson GL, Morgan DR, et al. 14-day triple, 5-day concomitant, and 10-day sequential therapies for Helicobacter pylori infection in seven Latin American sites: a randomised trial. Lancet 2011;378:507–514.



99. Nasa M, Choksey A, Phadke A, Sawant P. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a randomized study. Indian J Gastroenterol 2013;32:392–396.


100. Boal Carvalho P, Magalhães J, Dias de Castro F, Rosa B, Cotter J. Randomized controlled trial for Helicobacter pylori eradication in a naive portuguese population: is sequential treatment superior to triple therapy in real world clinical setting? Acta Med Port 2017;30:185–189.


101. Liou JM, Chen CC, Chang CY, et al. Sequential therapy for 10 days versus triple therapy for 14 days in the eradication of Helicobacter pylori in the community and hospital populations: a randomised trial. Gut 2016;65:1784–1792.


102. Phiphatpatthamaamphan K, Vilaichone RK, Siramolpiwat S, et al. Effect of IL-1 polymorphisms, CYP2C19 genotype and antibiotic resistance on Helicobacter pylori eradication comparing between 10-day sequential therapy and 14-day standard tripe therapy with four-times-daily-dosing of amoxicillin in Thailand: a prospective randomized study. Asian Pac J Cancer Prev 2016;17:1903–1907.

103. Alsohaibani F, Al Ashgar H, Al Kahtani K, et al. Prospective trial in Saudi Arabia comparing the 14-day standard triple therapy with the 10-day sequential therapy for treatment of Helicobacter pylori infection. Saudi J Gastroenterol 2015;21:220–225.



104. Liu KS, Hung IF, Seto WK, et al. Ten day sequential versus 10 day modified bismuth quadruple therapy as empirical firstline and secondline treatment for Helicobacter pylori in Chinese patients: an open label, randomised, crossover trial. Gut 2014;63:1410–1415.


105. Kefeli A, Basyigit S, Yeniova AO, Kefeli TT, Aslan M, Tanas O. Comparison of three different regimens against Helicobacter pylori as a first-line treatment: a randomized clinical trial. Bosn J Basic Med Sci 2016;16:52–57.



106. Sardarian H, Fakheri H, Hosseini V, Taghvaei T, Maleki I, Mokhtare M. Comparison of hybrid and sequential therapies for Helicobacter pylori eradication in Iran: a prospective randomized trial. Helicobacter 2013;18:129–134.


107. Chen KY, Lin TJ, Lin CL, Lee HC, Wang CK, Wu DC. Hybrid vs sequential therapy for eradication of Helicobacter pylori in Taiwan: a prospective randomized trial. World J Gastroenterol 2015;21:10435–10442.



108. Kim SY, Lee SW, Choe JW, et al. Helicobacter pylori eradication rates of concomitant and sequential therapies in Korea. Helicobacter 2017;22:e12441.

109. Molina-Infante J, Romano M, Fernandez-Bermejo M, et al. Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology 2013;145:121–128.e1.


110. Hong J, Shu X, Liu D, et al. Antibiotic resistance and CYP2C19 polymorphisms affect the efficacy of concomitant therapies for Helicobacter pylori infection: an open-label, randomized, single-centre clinical trial. J Antimicrob Chemother 2016;71:2280–2285.


111. Park SM, Kim JS, Kim BW, Ji JS, Choi H. Randomized clinical trial comparing 10- or 14-day sequential therapy and 10- or 14-day concomitant therapy for the first line empirical treatment of Helicobacter pylori infection. J Gastroenterol Hepatol 2017;32:589–594.


112. Liou JM, Fang YJ, Chen CC, et al. Concomitant, bismuth quadruple, and 14-day triple therapy in the first-line treatment of Helicobacter pylori : a multicentre, open-label, randomised trial. Lancet 2016;388:2355–2365.


113. Das R, Sureshkumar S, Sreenath GS, Kate V. Sequential versus concomitant therapy for eradication of Helicobacter pylori in patients with perforated duodenal ulcer: a randomized trial. Saudi J Gastroenterol 2016;22:309–315.



114. Georgopoulos SD, Xirouchakis E, Martinez-Gonzales B, et al. Randomized clinical trial comparing ten day concomitant and sequential therapies for Helicobacter pylori eradication in a high clarithromycin resistance area. Eur J Intern Med 2016;32:84–90.


115. Apostolopoulos P, Koumoutsos I, Ekmektzoglou K, et al. Concomitant versus sequential therapy for the treatment of Helicobacter pylori infection: a Greek randomized prospective study. Scand J Gastroenterol 2016;51:145–151.


116. Zhou L, Zhang J, Song Z, et al. Tailored versus triple plus bismuth or concomitant therapy as initial Helicobacter pylori treatment: a randomized trial. Helicobacter 2016;21:91–99.


117. Gungor G, Baglicakoglu M, Kayacetin E, et al. Current status of five different regimens for empiric first-line Helicobacter pylori eradication in Turkey. Digestion 2015;92:55–59.


118. Heo J, Jeon SW, Jung JT, et al. Concomitant and hybrid therapy for Helicobacter pylori infection: a randomized clinical trial. J Gastroenterol Hepatol 2015;30:1361–1366.


119. Cuadrado-Lavín A, Salcines-Caviedes JR, Diaz-Perez A, et al. First-line eradication rates comparing two shortened non-bismuth quadruple regimens against Helicobacter pylori : an open-label, randomized, multicentre clinical trial. J Antimicrob Chemother 2015;70:2376–2381.


120. Molina-Infante J, Lucendo AJ, Angueira T, et al. Optimised empiric triple and concomitant therapy for Helicobacter pylori eradication in clinical practice: the OPTRICON study. Aliment Pharmacol Ther 2015;41:581–589.


121. Ang TL, Fock KM, Song M, et al. Ten-day triple therapy versus sequential therapy versus concomitant therapy as first-line treatment for Helicobacter pylori infection. J Gastroenterol Hepatol 2015;30:1134–1139.


122. McNicholl AG, Marin AC, Molina-Infante J, et al. Randomised clinical trial comparing sequential and concomitant therapies for Helicobacter pylori eradication in routine clinical practice. Gut 2014;63:244–249.


123. Lim JH, Lee DH, Choi C, et al. Clinical outcomes of two-week sequential and concomitant therapies for Helicobacter pylori eradication: a randomized pilot study. Helicobacter 2013;18:180–186.


124. Georgopoulos S, Papastergiou V, Xirouchakis E, et al. Nonbismuth quadruple "concomitant" therapy versus standard triple therapy, both of the duration of 10 days, for first-line H. pylori eradication: a randomized trial. J Clin Gastroenterol 2013;47:228–232.


125. Wu DC, Hsu PI, Wu JY, et al. Sequential and concomitant therapy with four drugs is equally effective for eradication of H pylori infection. Clin Gastroenterol Hepatol 2010;8:36–41.e1.


126. De Francesco V, Pontone S, Bellesia A, et al. Quadruple, sequential, and concomitant first-line therapies for H. pylori eradication: a prospective, randomized study. Dig Liver Dis 2018;50:139–141.


127. De Francesco V, Hassan C, Ridola L, Giorgio F, Ierardi E, Zullo A. Sequential, concomitant and hybrid first-line therapies for Helicobacter pylori eradication: a prospective randomized study. J Med Microbiol 2014;63(Pt 5):748–752.


128. Huang YK, Wu MC, Wang SS, et al. Lansoprazole-based sequential and concomitant therapy for the first-line Helicobacter pylori eradication. J Dig Dis 2012;13:232–238.


129. Molina-Infante J, Pazos-Pacheco C, Vinagre-Rodriguez G, et al. Nonbismuth quadruple (concomitant) therapy: empirical and tailored efficacy versus standard triple therapy for clarithromycin-susceptible Helicobacter pylori and versus sequential therapy for clarithromycin-resistant strains. Helicobacter 2012;17:269–276.


130. Georgopoulos SD. "Concomitant" or "sequential" eradication of Helicobacter pylori : which regimen comes first? Ann Gastroenterol 2014;27:280–281.


131. Shin WG, Lee SW, Baik GH, et al. Eradication rates of Helicobacter pylori in Korea over the past 10 years and correlation of the amount of antibiotics use: nationwide survey. Helicobacter 2016;21:266–278.


132. Kim BJ, Kim HS, Song HJ, et al. Online registry for nationwide database of current trend of Helicobacter pylori eradication in Korea: interim analysis. J Korean Med Sci 2016;31:1246–1253.



133. Kim SG, Jung HK, Lee HL, et al. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. J Gastroenterol Hepatol 2014;29:1371–1386.


134. Wenzhen Y, Yumin L, Quanlin G, et al. Is antimicrobial susceptibility testing necessary before first-line treatment for Helicobacter pylori infection? Meta-analysis of randomized controlled trials. Intern Med 2010;49:1103–1109.


135. López-Góngora S, Puig I, Calvet X, et al. Systematic review and meta-analysis: susceptibility-guided versus empirical antibiotic treatment for Helicobacter pylori infection. J Antimicrob Chemother 2015;70:2447–2455.


136. Seo SI, Do BJ, Kang JG, et al. Helicobacter pylori eradication according to sequencing-based 23S ribosomal RNA point mutation associated with clarithromycin resistance. J Clin Med 2019;9:54.


137. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol 2017;112:212–239.

138. Lee JW, Kim N, Nam RH, et al. Favorable outcomes of culture-based Helicobacter pylori eradication therapy in a region with high antimicrobial resistance. Helicobacter 2019;24:e12561.


139. Li BZ, Threapleton DE, Wang JY, et al. Comparative effectiveness and tolerance of treatments for Helicobacter pylori : systematic review and network meta-analysis. BMJ 2015;351:h4052.



140. Yeo YH, Shiu SI, Ho HJ, et al. First-line Helicobacter pylori eradication therapies in countries with high and low clarithromycin resistance: a systematic review and network meta-analysis. Gut 2018;67:20–27.


141. Tsay FW, Wu DC, Yu HC, et al. A randomized controlled trial shows that both 14-day hybrid and bismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with moderate antibiotic resistance. Antimicrob Agents Chemother 2017;61:e00140–17.



142. Sapmaz F, Kalkan IH, Atasoy P, Basyigit S, Guliter S. A non-inferiority study: modified dual therapy consisting higher doses of rabeprazole is as successful as standard quadruple therapy in eradication of Helicobacter pylori. Am J Ther 2017;24:e393–e398.


143. Gokcan H, Oztas E, Onal IK. Different bismuth-based therapies for eradicating Helicobacter pylori : randomized clinical trial of efficacy and safety. Clin Res Hepatol Gastroenterol 2016;40:124–131.


144. Köksal AS, Onder FO, Torun S, et al. Twice a day quadruple therapy for the first-line treatment of Helicobacter pylori in an area with a high prevalence of background antibiotic resistance. Acta Gastroenterol Belg 2013;76:34–37.

145. Dore MP, Farina V, Cuccu M, Mameli L, Massarelli G, Graham DY. Twice-a-day bismuth-containing quadruple therapy for Helicobacter pylori eradication: a randomized trial of 10 and 14 days. Helicobacter 2011;16:295–300.


146. Songür Y, Senol A, Balkarli A, Baştürk A, Cerçi S. Triple or quadruple tetracycline-based therapies versus standard triple treatment for Helicobacter pylori treatment. Am J Med Sci 2009;338:50–53.


147. Wu TS, Hsu PI, Kuo CH, et al. Comparison of 10-day levofloxacin bismuth-based quadruple therapy and levofloxacin-based triple therapy for Helicobacter pylori. J Dig Dis 2017;18:537–542.


148. Munteanu D, Etzion O, Ben-Yakov G, et al. Efficacy and safety of sequential versus quadruple therapy as second-line treatment for Helicobacter pylori infection-a randomized controlled trial. PLoS One 2017;12:e0183302.



149. Mori H, Suzuki H, Matsuzaki J, et al. Rifabutin-based 10-day and 14-day triple therapy as a third-line and fourth-line regimen for Helicobacter pylori eradication: a pilot study. United European Gastroenterol J 2016;4:380–387.


150. Mori H, Suzuki H, Matsuzaki J, et al. Efficacy of 10-day sitafloxacin-containing third-line rescue therapies for Helicobacter pylori strains containing the gyrA mutation. Helicobacter 2016;21:286–294.


151. Liou JM, Bair MJ, Chen CC, et al. Levofloxacin sequential therapy vs levofloxacin triple therapy in the second-line treatment of Helicobacter pylori : a randomized trial. Am J Gastroenterol 2016;111:381–387.


152. Chuah SK, Liang CM, Lee CH, et al. A randomized control trial comparing 2 levofloxacin-containing second-line therapies for Helicobacter pylori eradication. Medicine (Baltimore) 2016;95:e3586.



153. Chen Q, Zhang W, Fu Q, et al. Rescue therapy for Helicobacter pylori eradication: a randomized non-inferiority trial of amoxicillin or tetracycline in bismuth quadruple therapy. Am J Gastroenterol 2016;111:1736–1742.


154. Yang JC, Lin CJ, Wang HL, et al. High-dose dual therapy is superior to standard first-line or rescue therapy for Helicobacter pylori infection. Clin Gastroenterol Hepatol 2015;13:895–905.e5.


155. Mansour-Ghanaei F, Joukar F, Naghipour MR, Forouhari A, Saadat SM. Seven-day quintuple regimen as a rescue therapy for Helicobacter pylori eradication. World J Gastroenterol 2015;21:661–666.



156. Jheng GH, Wu IC, Shih HY, et al. Comparison of second-line quadruple therapies with or without bismuth for Helicobacter pylori infection. Biomed Res Int 2015;2015:163960.



157. Cao Z, Chen Q, Zhang W, et al. Fourteen-day optimized levofloxacin-based therapy versus classical quadruple therapy for Helicobacter pylori treatment failures: a randomized clinical trial. Scand J Gastroenterol 2015;50:1185–1190.


158. Lim HC, Lee YJ, An B, Lee SW, Lee YC, Moon BS. Rifabutinbased high-dose proton-pump inhibitor and amoxicillin triple regimen as the rescue treatment for Helicobacter pylori. Helicobacter 2014;19:455–461.



159. Ierardi E, Giangaspero A, Losurdo G, et al. Quadruple rescue therapy after first and second line failure for Helicobacter pylori treatment: comparison between two tetracycline-based regimens. J Gastrointestin Liver Dis 2014;23:367–370.

160. Murakami K, Furuta T, Ando T, et al. Multi-center randomized controlled study to establish the standard third-line regimen for Helicobacter pylori eradication in Japan. J Gastroenterol 2013;48:1128–1135.


161. Moon JY, Kim GH, You HS, et al. Levofloxacin, metronidazole, and lansoprazole triple therapy compared to quadruple therapy as a second-line treatment of Helicobacter pylori infection in Korea. Gut Liver 2013;7:406–410.

162. Kuo CH, Hsu PI, Kuo FC, et al. Comparison of 10 day bismuth quadruple therapy with high-dose metronidazole or levofloxacin for second-line Helicobacter pylori therapy: a randomized controlled trial. J Antimicrob Chemother 2013;68:222–228.


163. Calhan T, Kahraman R, Sahin A, et al. Efficacy of two levofloxacin-containing second-line therapies for Helicobacter pylori : a pilot study. Helicobacter 2013;18:378–383.


164. Yoon JH, Baik GH, Kim YS, et al. Comparison of the eradication rate between 1- and 2-week bismuth-containing quadruple rescue therapies for Helicobacter pylori eradication. Gut Liver 2012;6:434–439.



165. Chuah SK, Tai WC, Hsu PI, et al. The efficacy of second-line anti-Helicobacter pylori therapy using an extended 14-day levofloxacin/amoxicillin/proton-pump inhibitor treatment--a pilot study. Helicobacter 2012;17:374–381.


166. Chuah SK, Hsu PI, Chang KC, et al. Randomized comparison of two non-bismuth-containing second-line rescue therapies for Helicobacter pylori. Helicobacter 2012;17:216–223.


167. Wu DC, Hsu PI, Tseng HH, et al. Helicobacter pylori infection: a randomized, controlled study comparing 2 rescue therapies after failure of standard triple therapies. Medicine (Baltimore) 2011;90:180–185.

168. Hu TH, Chuah SK, Hsu PI, et al. Randomized comparison of two nonbismuth-containing rescue therapies for Helicobacter pylori. Am J Med Sci 2011;342:177–181.


169. Gu LY, Lin WW, Lu H, Chen XY, Ge ZZ, Li XB. Quadruple therapy with medications containing either rufloxacin or furazolidone as a rescue regimen in the treatment of Helicobacter pylori-infected dyspepsia patients: a randomized pilot study. Helicobacter 2011;16:284–288.


170. Chung JW, Lee JH, Jung HY, et al. Second-line Helicobacter pylori eradication: a randomized comparison of 1-week or 2-week bismuth-containing quadruple therapy. Helicobacter 2011;16:289–294.


171. Minakari M, Davarpanah Jazi AH, Shavakhi A, Moghareabed N, Fatahi F. A randomized controlled trial: efficacy and safety of azithromycin, ofloxacin, bismuth, and omeprazole compared with amoxicillin, clarithromycin, bismuth, and omeprazole as second-line therapy in patients with Helicobacter pylori infection. Helicobacter 2010;15:154–159.


172. Lee BH, Kim N, Hwang TJ, et al. Bismuth-containing quadruple therapy as second-line treatment for Helicobacter pylori infection: effect of treatment duration and antibiotic resistance on the eradication rate in Korea. Helicobacter 2010;15:38–45.


173. Kuo CH, Wang SS, Hsu WH, et al. Rabeprazole can overcome the impact of CYP2C19 polymorphism on quadruple therapy. Helicobacter 2010;15:265–272.


174. Ueki N, Miyake K, Kusunoki M, et al. Impact of quadruple regimen of clarithromycin added to metronidazole-containing triple therapy against Helicobacter pylori infection following clarithromycin-containing triple-therapy failure. Helicobacter 2009;14:91–99.

175. Kuo CH, Hu HM, Kuo FC, et al. Efficacy of levofloxacin-based rescue therapy for Helicobacter pylori infection after standard triple therapy: a randomized controlled trial. J Antimicrob Chemother 2009;63:1017–1024.


176. Karatapanis S, Skorda L, Georgopoulos S, et al. Levofloxacinbased triple therapy versus bismuth-based quadruple therapy as a second line treatment for the eradication of H. pylori infection. Ann Gastroenterol 2009;22:263–267.
177. Di Caro S, Franceschi F, Mariani A, et al. Second-line levofloxacin-based triple schemes for Helicobacter pylori eradication. Dig Liver Dis 2009;41:480–485.

178. Bago J, Pevec B, Tomić M, Marusić M, Bakula V, Bago P. Second-line treatment for Helicobacter pylori infection based on moxifloxacin triple therapy: a randomized controlled trial. Wien Klin Wochenschr 2009;121:47–52.


179. Uygun A, Ozel AM, Yildiz O, et al. Comparison of three different second-line quadruple therapies including bismuth subcitrate in Turkish patients with non-ulcer dyspepsia who failed to eradicate Helicobacter pylori with a 14-day standard first-line therapy. J Gastroenterol Hepatol 2008;23:42–45.


180. Sanches B, Coelho L, Moretzsohn L, Vieira G Jr. Failure of Helicobacter pylori treatment after regimes containing clarithromycin: new practical therapeutic options. Helicobacter 2008;13:572–576.

181. Nishizawa T, Suzuki H, Nakagawa I, Iwasaki E, Masaoka T, Hibi T. Gatifloxacin-based triple therapy as a third-line regimen for Helicobacter pylori eradication. J Gastroenterol Hepatol 2008;23 Suppl 2:S167–S170.


182. Jung HS, Shim KN, Baik SJ, et al. Efficacy of levofloxacin-based triple therapy as second-line Helicobacter pylori eradication. Korean J Gastroenterol 2008;51:285–290.

183. Gisbert JP, Morena F. Systematic review and meta-analysis: levofloxacin-based rescue regimens after Helicobacter pylori treatment failure. Aliment Pharmacol Ther 2006;23:35–44.


184. Saad RJ, Schoenfeld P, Kim HM, Chey WD. Levofloxacinbased triple therapy versus bismuth-based quadruple therapy for persistent Helicobacter pylori infection: a meta-analysis. Am J Gastroenterol 2006;101:488–496.


185. Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol 2014;12:177–e13.


186. Marin AC, McNicholl AG, Gisbert JP. A review of rescue regimens after clarithromycin-containing triple therapy failure (for Helicobacter pylori eradication). Expert Opin Pharmacother 2013;14:843–861.


187. An B, Moon BS, Kim H, et al. Antibiotic resistance in Helicobacter pylori strains and its effect on H. pylori eradication rates in a single center in Korea. Ann Lab Med 2013;33:415–419.



188. Kim JM, Kim JS, Kim N, Jung HC, Song IS. Distribution of fluoroquinolone MICs in Helicobacter pylori strains from Korean patients. J Antimicrob Chemother 2005;56:965–967.


189. Lim JH, Kim SG, Song JH, et al. Efficacy of levofloxacin-based third-line therapy for the eradication of Helicobacter pylori in peptic ulcer disease. Gut Liver 2017;11:226–231.


190. Kang KK, Lee DH, Oh DH, et al. Helicobacter pylori eradication with moxifloxacin-containing therapy following failed first-line therapies in South Korea. World J Gastro enterol 2014;20:6932–6938.

191. Gisbert JP, Romano M, Gravina AG, et al. Helicobacter pylori second-line rescue therapy with levofloxacin- and bismuth-containing quadruple therapy, after failure of standard triple or non-bismuth quadruple treatments. Aliment Pharmacol Ther 2015;41:768–775.


192. Hsu PI, Wu DC, Chen A, et al. Quadruple rescue therapy for Helicobacter pylori infection after two treatment failures. Eur J Clin Invest 2008;38:404–409.


193. Yee YK, Cheung TK, Chu KM, et al. Clinical trial: levofloxacin-based quadruple therapy was inferior to traditional quadruple therapy in the treatment of resistant Helicobacter pylori infection. Aliment Pharmacol Ther 2007;26:1063–1067.


194. Gisbert JP, Calvet X. Review article: rifabutin in the treatment of refractory Helicobacter pylori infection. Aliment Pharmacol Ther 2012;35:209–221.


195. Miehlke S, Hansky K, Schneider-Brachert W, et al. Randomized trial of rifabutin-based triple therapy and high-dose dual therapy for rescue treatment of Helicobacter pylori resistant to both metronidazole and clarithromycin. Aliment Pharmacol Ther 2006;24:395–403.


196. Miehlke S, Kirsch C, Schneider-Brachert W, et al. A prospective, randomized study of quadruple therapy and high-dose dual therapy for treatment of Helicobacter pylori resistant to both metronidazole and clarithromycin. Helicobacter 2003;8:310–319.


197. Liou JM, Chen CC, Chen MJ, et al. Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet 2013;381:205–213.


198. Correa P, Fontham ET, Bravo JC, et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-Helicobacter pylori therapy. J Natl Cancer Inst 2000;92:1881–1888.


199. Leung WK, Lin SR, Ching JY, et al. Factors predicting progression of gastric intestinal metaplasia: results of a randomised trial on Helicobacter pylori eradication. Gut 2004;53:1244–1249.



200. Wong BC, Lam SK, Wong WM, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 2004;291:187–194.


201. Saito D, Boku N, Fujioka T, et al. Impact of H-pylori eradication on gastric cancer prevention: endoscopic results of the Japanese intervention trial (JITHP-study). A randomized multi-center trial. Gastroenterology 2005;128:A4.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart shows the selection process for key question 1.
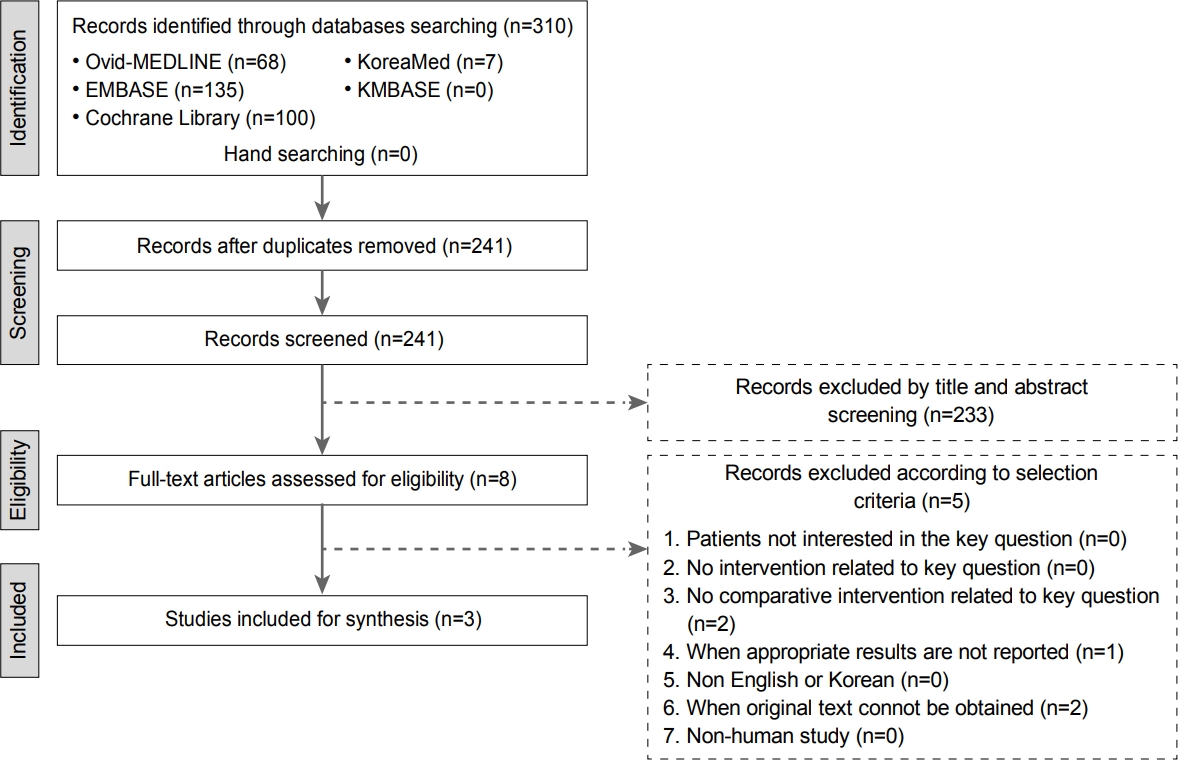
Figure 1.
Comparison of occurrence of metachronous gastric cancer after endoscopic resection of gastric adenoma between Hp eradication and placebo. Hp, Helicobacter pylori; M-H, Mantel-Haenszel test.
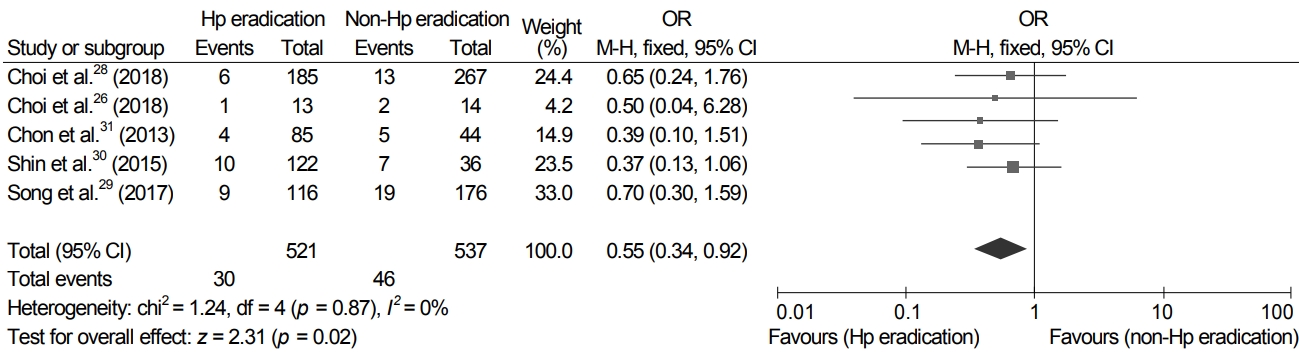
Figure 2.
Forest plot of studies reporting gastric cancer in the eradication group and control group from studies assessing the general population. M-H, Mantel-Haenszel test.
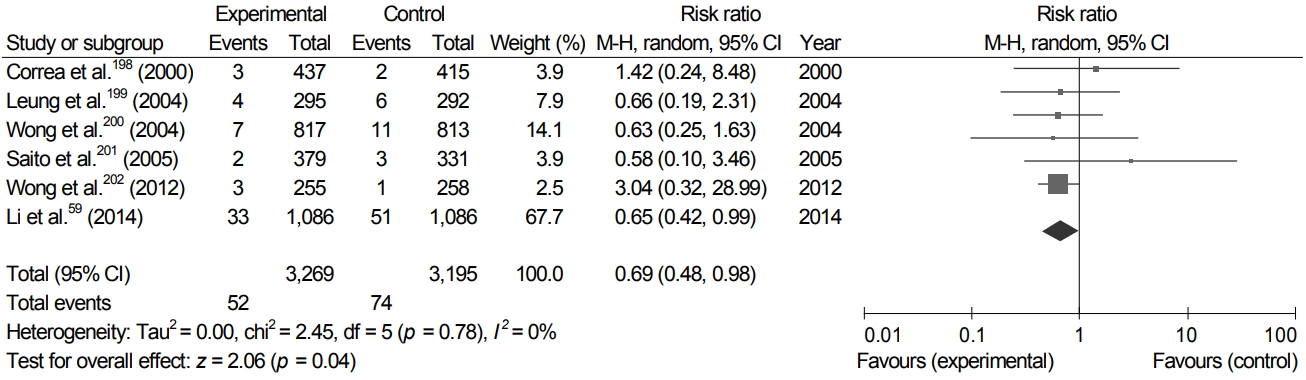
Figure 3.
Time trends of pooled Helicobacter pylori eradication rates with standard triple therapy based on data from randomized controlled trials performed in Korea. Overall eradication rates of standard triple therapy were 71.6% (95% CI 69.9-73.3%) in intention-to-treat (ITT) analysis and 79.6% (95% CI 76.6-82.2%) in per protocol (PP) analysis. Eradication rates from 2007 to 2011 were 72.3% (95% CI 71.2-74.4%) in ITT analysis and 81.5% (95% CI 79.9-82.9%) in PP analysis. Eradication rates from 2012 to 2016 were 70.3% (95% CI 68.4-72.1%) in ITT analysis and 77.4% (95% CI 75.6-79.2%) in PP analysis. ap<0.01.
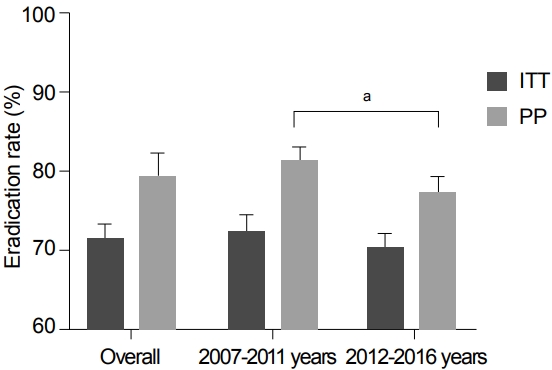
Figure 4.
Comparison of 10-day sequential therapy and standard triple therapy (TT) according to treatment duration of TT in intention-to-treat analysis. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bais); C, blinding of participants and personnel (performance bias); D, Blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); G, other bias. M-H, Mantel-Haenszel test.
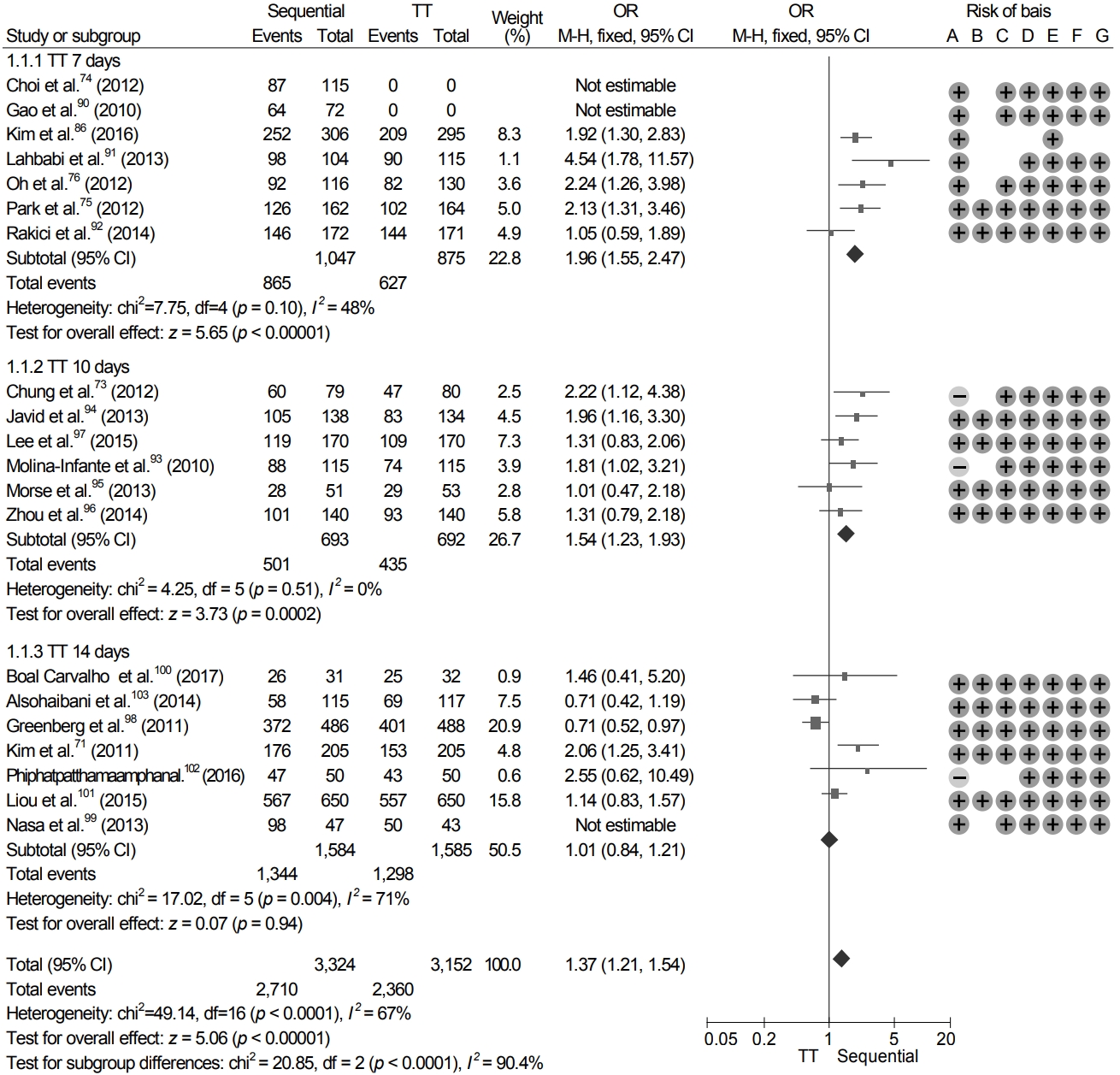
Figure 5.
Comparison of 10-day concomitant therapy (CT) and 10-/14-day standard triple (ST) therapy in intention-to-treat analysis. M-H, Mantel-Haenszel test.
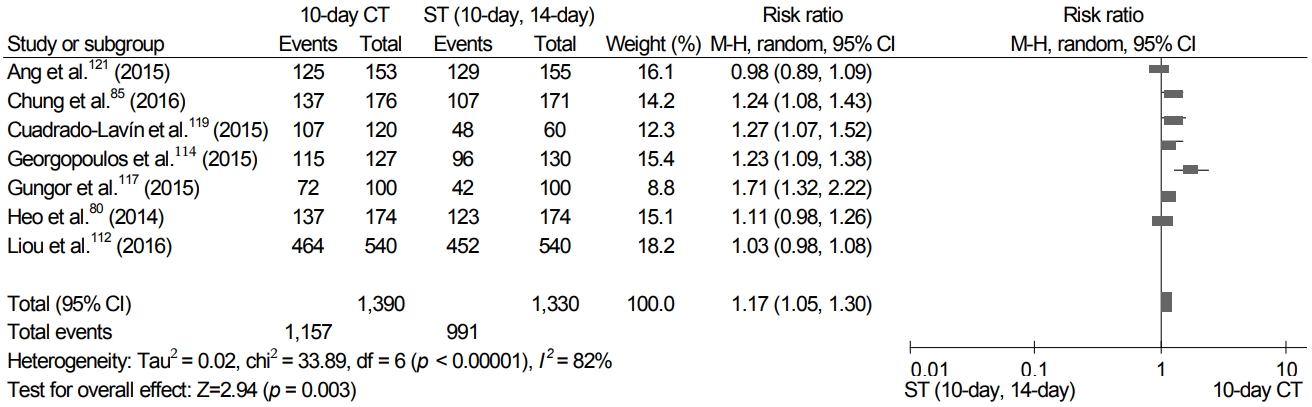
Figure 6.
Helicobacter pylori eradication rates with bismuth quadruple therapy as a first-line therapy. (A) Comparison of 10-/14-day bismuth quadruple therapy and 14-day standard triple therapy in intention-to-treat analysis. (B) Comparison of 10-/14-day bismuth quadruple therapy and 10-day sequential therapy in intention-to-treat analysis. (C) Comparison of 10-/14-day bismuth quadruple therapy and 10-day concomitant therapy in intention-to-treat analysis. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bais); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); G, other bias. PBMT, bismith quarduple therapy; M-H, Mantel-Haenszel test; SQT, sequential therapy; CCT, concomitant therapy.
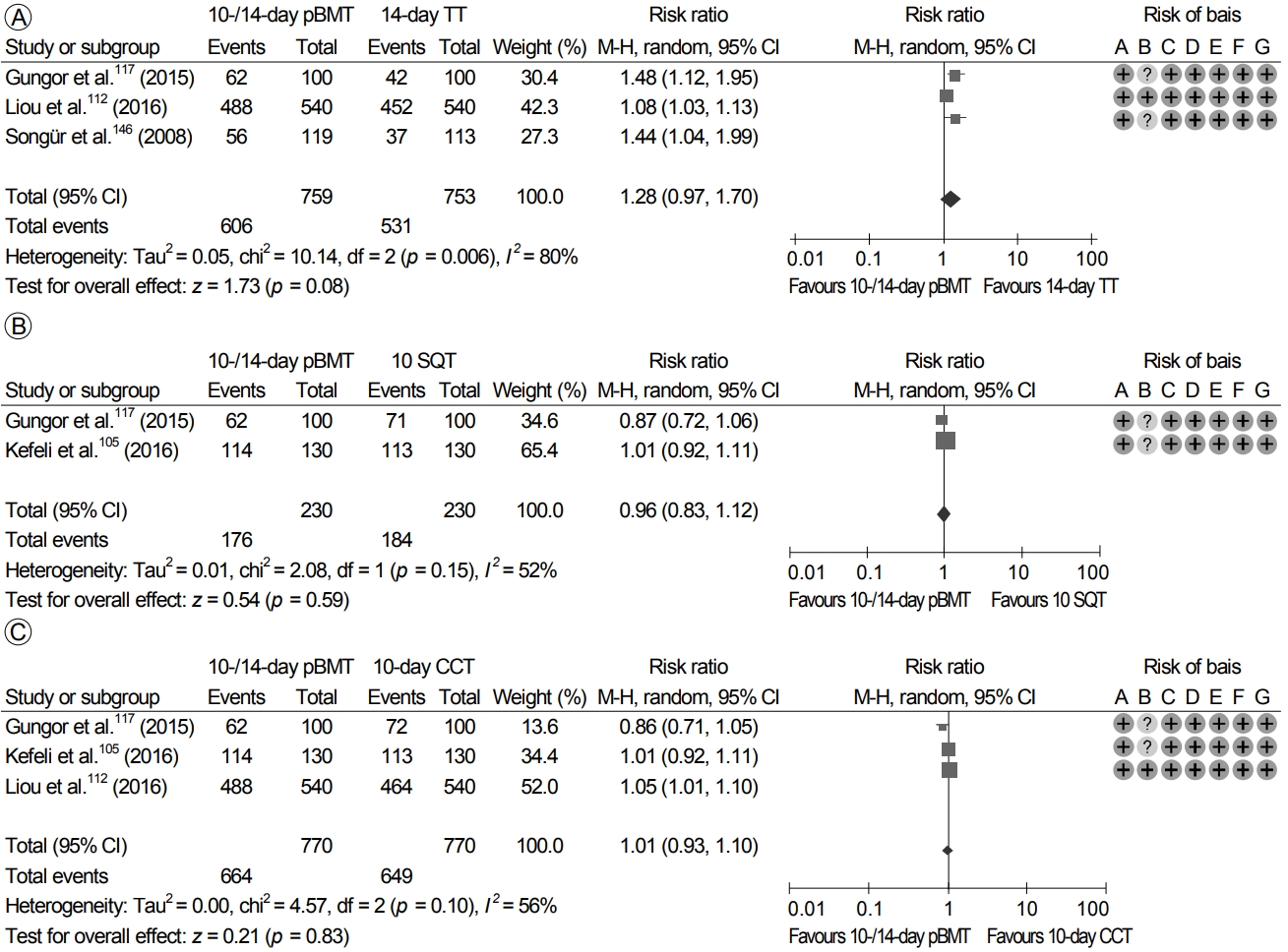
Figure 7.
Meta-analysis of nine studies that compare bismuth quadruple therapy with other regimens after the failure of first-line clarithromycin triple therapy. The pooled eradication rate of bismuth quadruple therapy as a second-line therapy is 75.5% (95% CI 71.6-79.1%).

Figure 8.
Meta-analysis of eight studies that compare levofloxacin triple therapy with other regimens after the failure of first-line clarithromycin triple therapy. The pooled eradication rate of levofloxacin triple therapy as a second-line therapy is 73.1% (95% CI 68.4-77.3%).
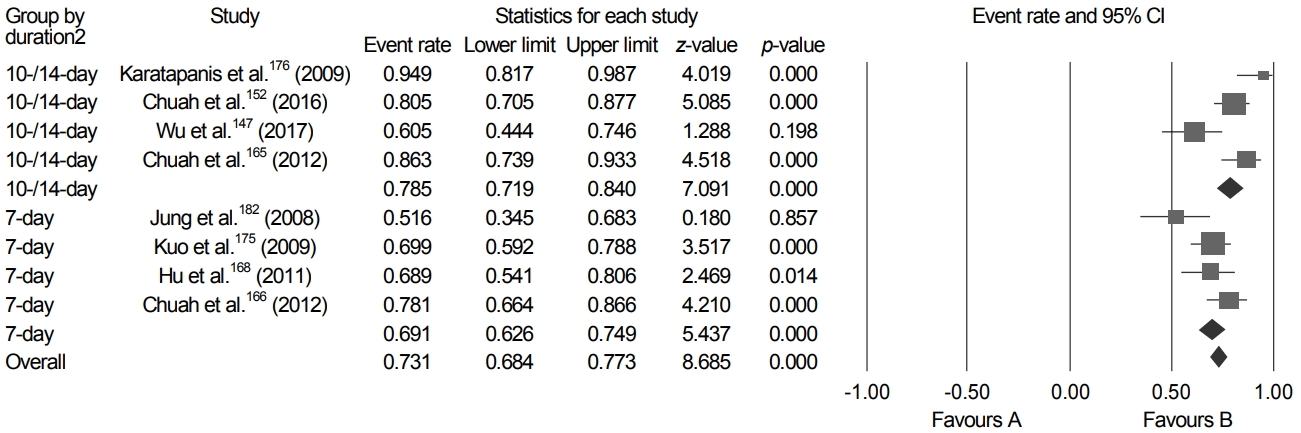
Figure 9.
Proposed algorithm for the treatment of Helicobacter pylori infection. Bismuth quadruple therapy as a first-line therapy is dotted because it is less preferred than other regimens. PPI, proton pump inhibitor.
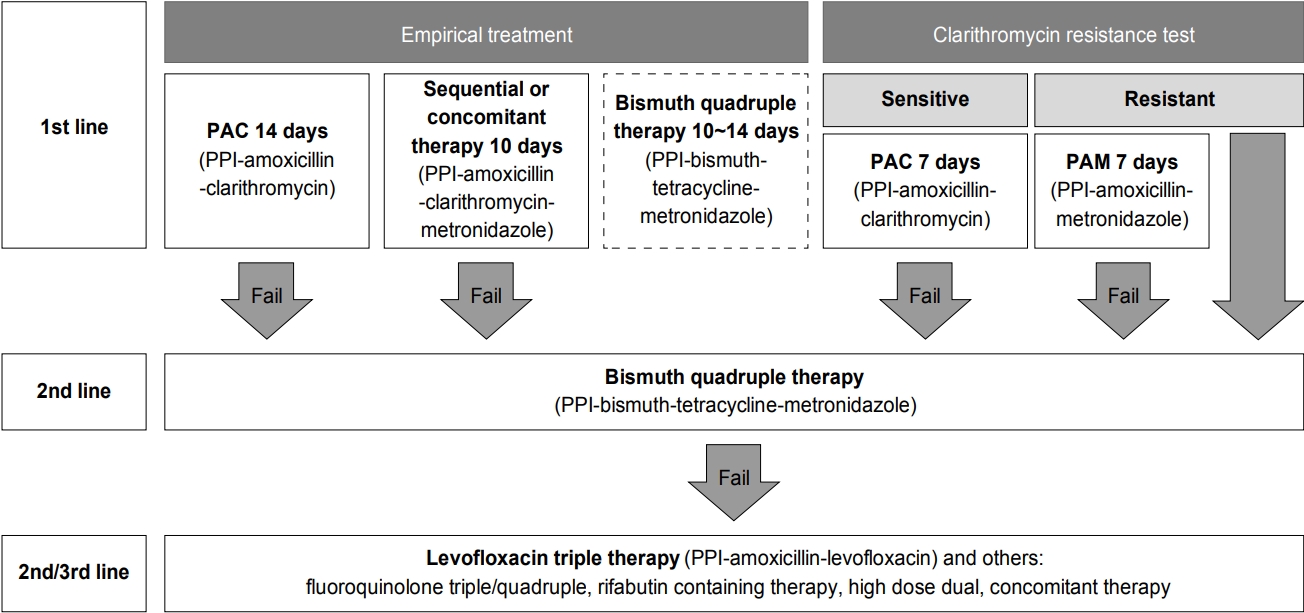
Figure 10.
Table 1.
Level of evidence and strength of recommendation
Table 2.
Recommendations for the treatment of H. pylori
Table 3.
Indication of the eradication of Helicobacter pylori
Table 4.
Regimen of recommended therapies for Helicobacter pylori infection
-
METRICS

-
- 1 Crossref
- 0 Scopus
- 16,847 View
- 991 Download



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



