INTRODUCTION
The predominant pathological findings of diabetic glomerulopathy include mesangial expansion, which may be nodular - the so-called Kimmelstiel-Wilson lesion - with thickening of the glomerular basement membrane and hyalinosis of the afferent and efferent arterioles [1]. However, whether the pathological findings of diabetic glomerulopathy can be used to predict kidney disease outcomes remains to be determined [2]. In routine clinical practice for the management of diabetes, diabetic nephropathy is usually diagnosed from clinical clues, and a kidney biopsy is reserved for patients with atypical presentations of glomerulonephritis and those with suspicion of other causes of glomerulonephritis [1]. Occasionally, other causes of glomerulonephritis may be encountered in diabetes patients, with or without diabetic nephropathy [3]. One report indicates that crescents, as a pathological component of pauci-immune and rapidly progressive glomerulonephritis, may appear in the glomeruli of diabetic patients with or without diabetic glomerulopathy [4]. However, glomerular crescents have rarely been reported in patients with diabetic glomerulopathy [5,6]. We present an unusual case of newly diagnosed type 2 diabetes with an accelerated course of nephropathy, who had advanced diabetic glomerulosclerosis with crescents at the time of diagnosis.
CASE REPORT
A 34-year-old man was admitted with generalized edema. He had no medical history but his mother suffered from diabetes mellitus. He had noted foamy urine for a year. His body had started to swell 1 month previously and this had worsened during the previous week after he caught a cold. His blood pressure was 150/100 mmHg at presentation. His random serum glucose level was 471 mg/dL (reference range, 70-140 mg/dL) with high hemoglobin A1C 11.3% (reference range 4.4-6.4%); thus, type 2 diabetes was diagnosed. Serum creatinine and blood urea nitrogen were elevated at 2.4 mg/dL (reference range, 0.5-1.4 mg/dL) and 36 mg/dL (reference range, 7-20 mg/dL), respectively. No urinary symptom was seen. Total protein and albumin had fallen to 5.0 g/dL (reference range, 6.4-8.5 g/dL) and 2.0 g/dL (reference range, 3.2-5.5 g/dL), respectively, and cholesterol had increased to 287 mg/dL. In the initial urine, many RBC/HPF and 5-9 WBC/HPF were found. Urinary RBCs were seen at 5-9/HPF throughout the follow-up samples, and the urinary WBCs disappeared. A 24-h urine test collected 5,842 mg/day of protein, and creatinine clearance was measured at 37.4 mL/min. Tests for anti-neutrophil cytoplasmic antibodies (ANCA), anti-glomerular basement membrane antibody, cryoglobulin, hepatitis B virus surface antigen, anti-hepatitis B surface antigen antibody, anti-hepatitis C virus antibody, and anti-human immunodeficiency virus (HIV) antibody were all negative. Anti-glutamic acid decarboxylase antibody and islet cell antibody were negative; thus, the possibility of an autoimmune cause of the diabetes was ruled out. Mild pulmonary edema was observed on chest X-ray examination. There was no specific finding, except edematous changes, on ultrasonography. Proliferative diabetic retinopathy was found in an ophthalmological examination, with no evidence of hypertensive retinopathy.
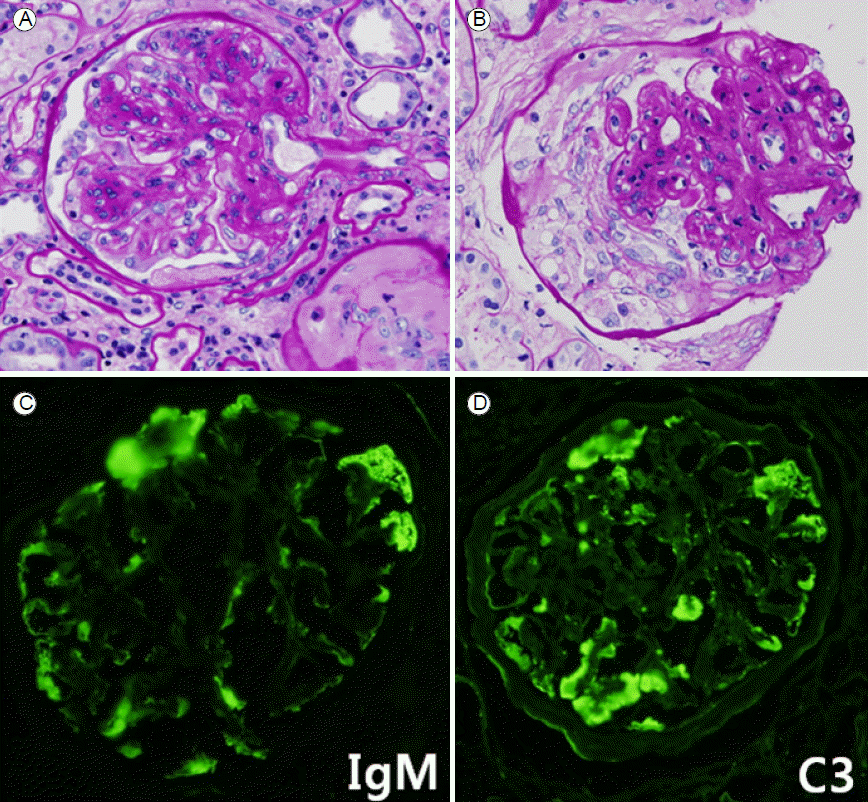
Proteinuria was in the nephrotic range and was combined with pyuria, microscopic hematuria, and markedly decreased renal function; thus, a renal biopsy was performed. The biopsy contained 20 glomeruli, one was globally sclerotic and two had cellular crescents. Most glomeruli were markedly increased in size and cellularity, with mesangial expansion. Periglomerular fibrosis was observed; no glomerulus showed partial sclerosis. The mesangium was greatly expanded with a diffuse increase of mesangial matrix and intercapillary sclerosis with segmental acellular mesangial nodules (Kimmelstiel-Wilson nodules; Fig. 1). In trichrome staining, a moderate level of tubular atrophy and lymphocyte infiltration was found in the interstitium, with a moderate level of fibrosis. Thus, staining indicated only a moderate level of chronic inflammation. There was no evidence of end-stage renal disease or acute inflammation of tubules and interstitium, as can be found in primary glomerular diseases. There were also large insudations in the subendothelial regions, forming a fibrin cap. In the blood vessels, hyaline arteriosclerosis was seen without vasculitis or fibrinoid necrosis. Immunofluorescence analysis revealed no significant immune deposits. No cellular crescent or glomerular basement membrane rupture was seen in the electron microscopy sample. Histological findings were consistent with diabetic nephropathy class III, in accordance with the criteria of the Renal Pathology Society[1]. The presence of cellular crescents generally leads to suspicion of pauci-immune crescentic glomerulonephritis, superimposed on an underlying diabetic glomerulosclerosis. However, we presumptively diagnosed this case as diabetic glomerulosclerosis with aberrant crescent formation, on the basis of serum ANCA negativity, the absence of necrotizing features in glomeruli, and no evidence of vasculitis.
An attempt was made to slow the progression of nephropathy with strict control of glucose and blood pressure, with insulin, an angiotensin receptor blocker, and a calcium channel blocker. However, over a 9-month period, serum creatinine increased gradually from 2.4 to 6.3 mg/dL. Proteinuria never fell below 5 g/day; maintenance hemodialysis was initiated due to the development of pericardial effusion, considered a uremic symptom.
DISCUSSION
We have described a case of newly diagnosed type 2 diabetes already displaying advanced glomerulopathy and cellular crescents, with rapid decline of renal function to the end stage. The unusual finding of crescents in the diabetic nephropathy, together with typical findings of nodular sclerosis, was in contrast to the occurrence of inflammatory crescents in the vasculitis.
In 1975, Elfenbein and Reyes [5] reported finding crescentic glomeruli in cases of diabetic glomerulosclerosis in autopsy and biopsy specimens: 43 findings among 5,696 glomeruli in autopsies, and 16 among 253 glomeruli in biopsies. They also observed that the presence of crescents in diabetic glomerulosclerosis was correlated with the level of creatinine and the extent of proteinuria. In the United States, Nasr et al. [4] reported the clinical characteristics of 23 cases of crescentic glomerulonephritis in diabetic glomerulonephritis; in four cases, the patients were under the age of 50, seven cases occurred less than 10 years after the diagnosis of diabetes, four cases were ANCA-negative, and 15 had no extra-renal manifestations of vasculitis. The authors did not suggest that the cause of crescents may, in some of the cases, be due to diabetes [4]. In 2012, Otani et al. [6] reported a case resembling the present one. A 53-year-old male who had suffered from type 2 diabetes for 11 years presented with generalized edema with decreased renal function and massive proteinuria. In a biopsy, 9 of 17 glomeruli contained crescents on a background of diabetic glomerulosclerosis. No clinical clues suggested any alternative cause of the crescents. However, despite similar biopsy findings, the clinical features differed from our case, because the nephropathy was found 11 years after the diagnosis of diabetes, indicating a very slow progression.
The Renal Pathology Society classifies diabetic nephropathy according to the type of glomerular lesion. The four classes are: thickening of the basement membrane, mesangial expansion, nodular sclerosis (Kimmelstiel-Wilson), and fibrin cap. Interstitial fibrosis, tubular atrophy, and hyaline arteriosclerosis can be seen accompanying the glomerular changes. However, there is no reference to glomerular crescent formation in diabetic nephropathy [1].
Recent work suggests that crescents in diabetes may reflect a process of recovery from podocyte injury, involving the proliferation of parietal epithelial cells, although the pathogenesis of crescent formation in diabetic glomerulosclerosis has not been definitively established. Gaut et al. [7] characterized the cellular composition of glomerular crescents in diabetic nodular glomerulosclerosis and compared the expression of nephrin and claudin 1 with that in glomeruli containing inflammatory crescents, diabetic glomeruli without crescents, and normal- appearing glomeruli. Crescentic cells in diabetes expressed claudin 1 or nephrin. Nephrin-positive cells in diabetic crescents were significantly increased in comparison with inflammatory crescents, whereas there was a decrease in the glomerular tuft in diabetes, with or without crescents. Lower podocyte number may predict the development and progression of diabetic nephropathy [8,9]. Although podocyte injury plays an important role in the pathogenesis of diabetic nephropathy, it is not known why glomerular crescents in diabetic nephropathy have been observed only rarely. One possible explanation is that kidney biopsies in diabetes are reserved for patients with atypical presentations and other primary glomerular diseases.
The case presented is an unusual example of diabetic nephropathy in which the patient suffered from advanced nephropathy at the time when diabetes was diagnosed, and which progressed rapidly to the end stage, requiring dialysis. The cellular crescents, which accompanied nodular sclerosis, may hold the key to this extraordinary clinical feature. Further efforts, such as exploring the relationship between crescent formation and diabetic glomerulosclerosis, are needed to uncover the mechanism of this non-inflammatory crescent formation. The features of nephropathy in type 2 diabetes are quite heterogeneous, which can sometimes be confusing to clinicians. We suggest that renal biopsies should be performed without delay when type 2 diabetic nephropathy progresses unusually rapidly or has extraordinary features, not only to rule out the possibility of other causes but also to try to identify related factors that may be involved. This type of approach may facilitate progress in understanding the pathophysiology of rapidly progressive subtypes of diabetic nephropathy and in formulating prognoses.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print






