간세포암으로 오인된 간 전이를 동반한 위장관신경내분비종양
Gastroenteropancreatic Neuroendocrine Tumor with Hepatic Metastasis Misdiagnosed as Hepatocellular Carcinoma
Article information
Trans Abstract
The liver is the most common site of metastasis of neuroendocrine tumors (NETs). Gastroenteropancreatic (GEP)-NETs are rare, and the distinction between hepatocellular carcinoma (HCC) and metastatic NET can be difficult due to the similarity of their histological characteristics. Herein, we report a case of GEP-NET with hepatic metastasis, which was first misdiagnosed as HCC by liver biopsy and subsequently re-diagnosed after surgery as primary GEP-NET.
INTRODUCTION
Neuroendocrine tumors (NETs) originate from heterogenous neuroendocrine cells of various organs, such as the pancreas, gastrointestinal tract, and lung, as well as the parathyroid, adrenal, and pituitary glands or parafollicular C cells of the thyroid gland.
The overall annual age-adjusted incidence of NETs is 6.98/100,000 in the United States, according to the surveillance, epidemiology, and end results (SEER) registry. The incidence of NET is 3.56/100,000 persons, affecting a variety of gastroenteropancreatic (GEP) sites (including 1.05/100,000 persons with small-intestinal NET, 1.04/100,000 persons with rectal NET, and 0.48/100,000 persons with pancreatic NET) [1]. In the Asian population, the incidence of NET was reportedly 2.2/100,000 in 2004, and the proportion of NETs with a GEP origin was 58.2% in the rectum, 11.4% in the pancreas, 9.5% in the stomach, 5.6% in the duodenum, 5.6% in the jejunum/ileum, 7.0% in the colon, and 2.8% in the appendix [2]. In a multicenter study of GEP-NET in South Korea, the annual incidence of GEP-NET was found to have increased significantly from 2000 and 2009, and the rates of NET in specific organs differed compared to previous Western reports (48.0% in the rectum, 14.6% in the stomach, 8.7% in the pancreas, 7.7% in the small intestine, 7.9% in the colon, and 2.5% in the appendix) [3].
Fewer NETs originate from the small intestine than from other organs, and such NETs typically show an indolent clinical course. The nonspecific clinical symptoms often delay the diagnosis of NETs, and distant metastases are commonly detected at the time of initial diagnosis.
The liver is the most common site of GEP-NET metastases, and hepatic metastases are the most powerful predictor of survival in patients with GEP-NET. However, distinguishing between hepatocellular carcinoma (HCC) and NET metastases to the liver can be challenging, depending on the biopsy size, as some metastatic NETs may have similar cytological characteristics, such as increased cytoplasm, presence of nucleoli, and lack of salt-and-pepper chromatin, which may cause confusion with HCC [4,5]. Here, we report a case of GEP-NET with hepatic metastasis, which was first misdiagnosed as HCC by liver biopsy but was re-diagnosed after surgery as primary GEP-NET.
CASE REPORT
A 73-year-old man visited a local clinic due to discomfort in both hands and legs. The findings of initial spine magnetic resonance imaging (MRI) were normal, but the results of initial laboratory tests showed hypokalemia, and the patient had a history of hypertension. Thus, he was referred to a specialist for a hyperaldosteronism workup and was diagnosed with hyperaldosteronism due to detection of a left adrenal mass by abdominal computed tomography (CT), which also detected multiple hepatic masses. The patient had no definite history of hepatitis B or C, and he was negative for other hepatitis markers, except for HBcAb, and the level of the tumor marker alpha-fetoprotein (AFP) was within the normal range (1.82 ng/mL). Thus, the patient underwent liver biopsy at a local hospital, resulting in a diagnosis of HCC. The patient wished to have his HCC treated in a more advanced hospital; thus, he was referred to Kangbuk Samsung Hospital.
We attempted to re-biopsy the hepatic mass on hepatic segment 4 for accurate diagnosis despite the diagnosis of HCC of Edmondson-Steiner grade 2 (Fig. 1). The HCC was classified as T3N0M0 and BCLC stage B. The patient underwent seven sessions of transarterial chemoembolization (TACE) and one of radiofrequency ablation (RFA) for 3 years beginning in November 2015. The treatment was successful and the patient was followed up at the outpatient department by liver imaging and assessment of the levels of tumor markers until January 2018.
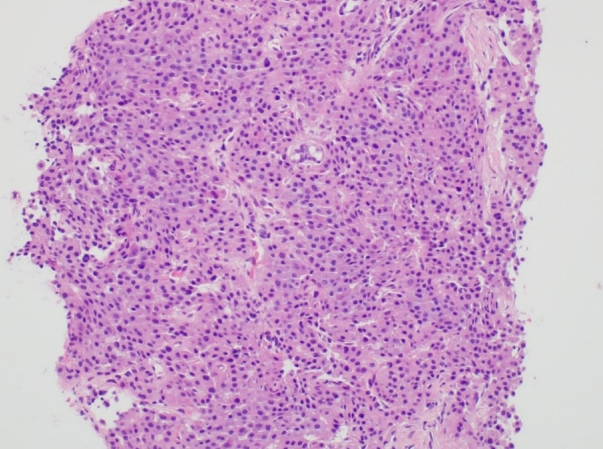
Ultrasound-guided needle biopsy of the hepatic mass showing hepatocellular carcinoma of Edmondson-Steiner grade 2 (hematoxylin and eosin [H&E], ×200).
After 1 month, the patient visited a local emergency department due to abrupt-onset abdominal pain, nausea, and vomiting. His vital signs were stable but emergent abdominal CT revealed a new mass on the ileocecal valve and several hepatic nodules, which was confirmed by positron emission tomography-CT (PET-CT) (Fig. 2). He was again referred to Kangbuk Samsung Hospital for further evaluation, and the initial laboratory findings showed elevated serum levels of C-reactive protein, hypokalemia (2.6 mEq/L potassium), and hypoalbuminemia (3.3 g/dL serum albumin). Abdominal X-ray showed no definite stepladder pattern of the ileus. We decided to perform a colonoscopy to evaluate the mass on the ileocecal valve found in the previous abdominal CT. Colonoscopy showed an edematous ileocecal valve with no definite mass-like lesion, but with several nodular lesions on its surface. Finally, biopsy of the ileocecal valve demonstrated that the nodules were NETs as confirmed by positive immunohistochemical staining for CK and CD56, with no hepatocytes (Fig. 3).
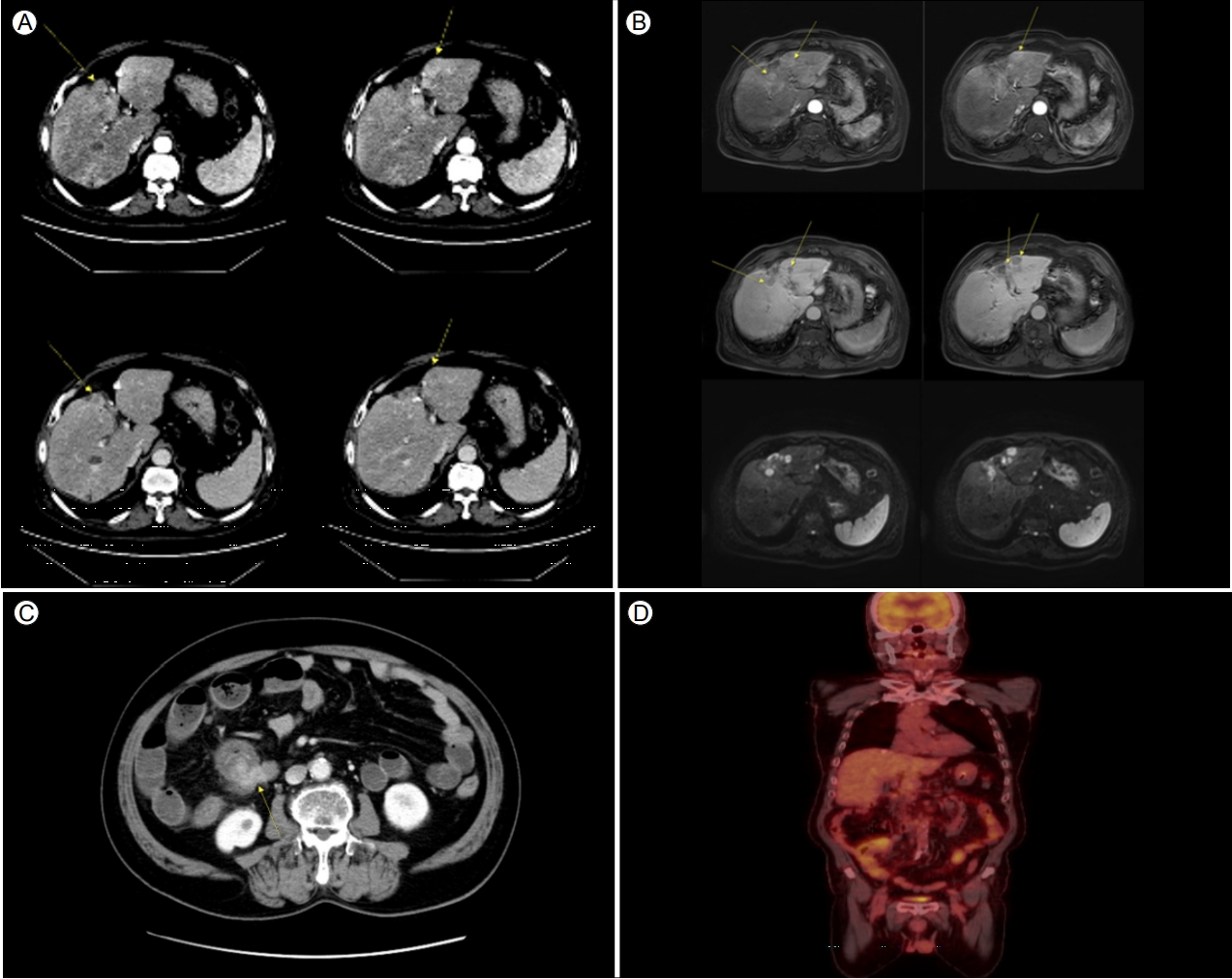
(A) Abdominal computed tomography (CT) scan demonstrating multiple hepatic nodules and an arterial enhancing and delayed wash-out pattern (arrows). (B) The same lesions are demonstrated by liver-dynamic magnetic resonance imaging (MRI), showing arterial enhancing, delayed wash-out, and diffusion restriction (arrows). (C) Abdominal CT scan demonstrating lesions due to nodular enhancement in the cecal and pericecal areas (arrow). (D) Tumor indicated by fluorodeoxyglucose (FDG) uptake by positron emission tomography-CT (PET-CT) scan.

(A) Colonoscopy demonstrating an edematous ileocecal valve. (B) Several nodular lesions on the surface of the ileocecal valve. (C) Endoscopic biopsy of an ileocecal valve lesion demonstrating neuroendocrine tumor (H&E, ×200) and positive immunohistochemical staining for CK and CD56 (×200).
After colonoscopic diagnosis, the symptoms were aggravated, and mechanical ileus was observed. We decided to proceed with surgery to resolve the ileocecal valve problem, in consultation with a surgeon. The patient underwent a right hemicolectomy with hand-assisted laparoscopic surgery (HALS). During the surgery, one of the hepatic nodules was resected to evaluate the presence of HCC or hepatic metastasis of NETs. The ileocecal valve tumor was composed of two cell populations: small cells and large polygonal cells with a trabecular and solid pattern. The small cells had small round nuclei, fine granular chromatin, and a predominant trabecular pattern, which are consistent with classic NET. By contrast, the large cells had abundant eosinophilic cytoplasm, vesicular nuclei with prominent nucleoli, and a trabecular and solid arrangement. Such histologic features indicated the possibility of HCC. Immunohistochemical stains were performed to rule out the presence of metastatic NET. The hepatic tumor cells were positive for the neuroendocrine marker CD56 and negative for hepatocyte antigen and glypican-3, although the histologic features wer suggestive of HCC. The tumor cells in the intestine exhibited the same immunohistochemical features. Therefore, the tumor cells in the liver were presumed to be metastases of large cells from intestinal NETs (Fig. 4).

(A) Ileocecal valve tumor composed of two cell populations: small cells and large polygonal cells with a trabecular and solid pattern (H&E, ×100). (B) Small cell compartment consistent with classic neuroendocrine tumor (H&E, ×200). (C) Large cell compartment showing histologic features, suggestive of hepatocellular carcinoma (H&E, ×200). (D) Immunohistochemical staining of the ileocecal valve area: positive for neuroendocrine markers (chromogranin, CD56, synaptophysin) and negative for hepatocyte antigen and glypican-3 (×200). (E) Hepatic mass resected during hemicolectomy demonstrating the same histologic characteristics (large cell population) and results of immunohistochemical staining as the ileocecal valve tumor (H&E, ×200).
These results were confusing as the resected hepatic nodule was first diagnosed as HCC by liver imaging. We thus reviewed the previous imaging and pathological findings to ensure that the hepatic mass was first diagnosed as HCC. After discussion with a pathologist and repeat immunohistochemical staining of the first biopsy specimen, we found that the first biopsied hepatic mass was actually a NET, not HCC (Fig. 5). Because the first pathological analyses were suggestive of HCC and the patient had a history of positivity for HBcAb, no further immunohistochemical staining for NET was performed and the hepatic mass was initially diagnosed as HCC.

Liver biopsy specimen, which was initially diagnosed as HCC, showing positive results of immunohistochemical staining for (A) chromogranin, (B) CD56, and (C) synaptophysin, demonstrating that the hepatic mass is a metastatic neuroendocrine tumor, not hepatocellular carcinoma (×100).
We assessed the levels of other markers of NET, such as 5-hydroxyindoleacetic acid (5-HIAA), and found an increased concentration of 5-HIAA in serum and 24 hours urine. Accordingly, the patient was diagnosed with gastrointestinal NET with hepatic metastasis during the third year of follow-up.
DISCUSSION
Differentiation between NET with hepatic metastasis and HCC is complicated due to their similar pathological features. Thus, a detailed history focusing on risk factors of HCC or NET is important. In our case, the patient had no definite symptoms of NET, such as flushing or diarrhea, and so NET was not suspected. However, the patient also had no risk factors for HCC, such as underlying liver disease or markers of viral infection, except for being positive for HBcAb; therefore, the physicians should have been more careful when diagnosing HCC. In addition, the initial levels of markers of HCC (such as AFP) were normal, which was not in agreement with the detection of multiple tumors by imaging; this should have raised suspicion. Thus, we performed a second liver biopsy, which confirmed that a misdiagnosis had occurred, likely due to the similarity of HCC and NET. The last hepatic resection specimen showed two patterns of cells: one with a hepatoid architecture and another with a NET-like architecture, as indicated by immunohistochemical staining. The first biopsy result showed only the former, which hampered led to a diagnosis of HCC rather than NET.
NET can be diagnosed by immunochemical staining for neuroendocrine markers, such as neuron-specific enolase (NSE) and protein gene product 9.5 (PGP 9.5), which are commonly expressed by NETs. However, immunochemical staining is performed only on specimens suspicious for NET, for reasons of cost-effectiveness. This can be a problem because NETs and HCCs often display similar (and confounding) cytological characteristics, such as increased cytoplasm, presence of nucleoli, lack of salt-and-pepper chromatin, and a growth pattern involving ovoid or spindle cells arranged in a trabecular, nodular, or pseudoglandular pattern divided by sinusoids. Such similarities can also be found by other approaches such as liver dynamic CT or MRI. Several cases of NET were misdiagnosed as HCC after retrograde review [6]. Therefore, a diagnosis of metastatic NET in the liver must be presumed when plasmacytoid cells are intermingled with broad bands of connective tissue, or associated with delicate strands of fibrovascular tissue. If there is any doubt, these neoplasms must be distinguished based on the results of immunohistochemical staining.
The treatment of gastrointestinal NET with hepatic metastasis, which is not suitable for curative surgery, is usually the same as for HCC, which includes TACE or RFA, according to the current guidelines [7]. Somatostatin analogs, such as octreotide, can be considered if systemic symptoms induced by distant metastatic lesions are present. Kwekkeboom et al. [8] reported favorable response rates and progression-free survival in patients with inoperable or metastatic GEP-NET upon treatment with the radiolabeled somatostatin analogue octreotide, with few side effects. The effectiveness of surgical interventions is a matter of debate, although two studies have reported that surgery is effective for primary GEP-NET with distant metastases [9,10]. In our case, because the patient had an elevated serum concentration of 5-HIAA, systemic therapy with a somatostatin analog or chemotherapy after removal of the primary GEP-NET was considered.
In summary, we present a case of GEP-NET with hepatic metastasis that was first misdiagnosed as HCC by liver biopsy, and later re-diagnosed as NET by immunochemical staining. It is important to be aware that a hepatic mass can be a primary hepatic carcinoma or a metastatic lesion originating at an extrahepatic site. If any biopsy or imaging result is suggestive of NET, measurements of the levels of tumor biomarkers in blood, as well as immunochemical staining, must be performed. Accurate diagnosis and appropriate treatment depend on a multidisciplinary assessment with close cooperation among physicians, surgeons, radiologists, and pathologists.