다발성 이시성 암: 5중복암 1예
Multiple Metachronous Malignancies: One Patient with Five Primary Malignancies
Article information
Trans Abstract
We report a case of a 73-year-old male with multiple, metachronous primary malignancies. He presented with adenocarcinoma of the stomach with transverse colon invasion followed by bladder cancer, hypopharyngeal cancer, urothelial cancer, and hepatocellular carcinoma, in that order, over 10 years. While these multiples malignancies were separate entities, they shared several etiologic factors, including smoking. To the best of our knowledge, this is the first description of five metachronous malignancies in a Korean patient.
INTRODUCTION
Multiple primary malignancies (MPMs) are defined as those that are determined to be distinct entities and of non-metastatic origin [1]. They are classified as synchronous when they are identified within the first 6 months after the appearance of the first lesion, or as metachronous when they are identified thereafter [1,2]. MPMs have been diagnosed with increasing frequency during the past decade [3]. The overall prevalence of MPMs is 0.7-11.7% and it increases proportionally with age [1,4,5]. Several other factors are responsible for the development of MPMs, including genetic predisposition and immune and environmental factors, such as chemicals, nutrition, viruses, chemotherapeutic regimens, and ionizing radiation [6,7]. Exposure to carcinogens can target different organs at the same time; for example, smoking can affect the lungs, nasopharynx, and bladder while human papilloma virus is associated with cancers of the vulva, vagina, and cervix [8]. In some cases, MPMs may arise by chance, as successful treatment of one malignancy prolongs survival, increasing the probability of the occurrence of a second malignancy. Some case reports have implicated a common trigger in the pathogenesis of MPMs [4,9-11], but the etiology of MPMs is poorly understood. Here we report a case of a Korean patient with five primary malignant tumors that developed over an 11-year period and had a common trigger in their pathogenesis.
CASE REPORT
The patient was a 73-year-old Korean male with a history of diabetes mellitus, hypertension and alcoholic liver cirrhosis. He had smoked 20 cigarettes/day for 40 years and drank heavily for 50 years. His family history was negative for malignancy. He presented with dyspnea on exertion during his first visit (2003). His blood pressure was 160/80 mmHg; his heart rate was 92 beats/min; his body temperature was 36.4℃, and his respiratory rate was 16 breaths/min.
His chest radiograph was normal. He had a hemoglobin level of 4.3 g/dL, white blood cell count of 6.9 × 109/L, platelet count of 178 × 109/L, potassium level of 3.0 mmol/L, calcium level of 7.6 mg/dL, protein level of 5.6 g/dL, and albumin level of 3.0 g/dL. His blood sugar was normal, as was his liver and kidney function. Tumor marker levels were normal, except for carcinoembryonic antigen (CEA) (9.91 ng/mL). Serological testing was negative for HBsAg, HBsAb, and anti-HCV.
Esophagogastroduodenoscopy showed a huge ulcerofungating mass involving the antrum and lower body of the stomach, leading to obstruction of the pyloric ring (Fig. 1). The histological diagnosis was moderately differentiated adenocarcinoma of the stomach with transverse colon invasion. The patient underwent a subtotal gastrectomy with segmental resection of the colon.
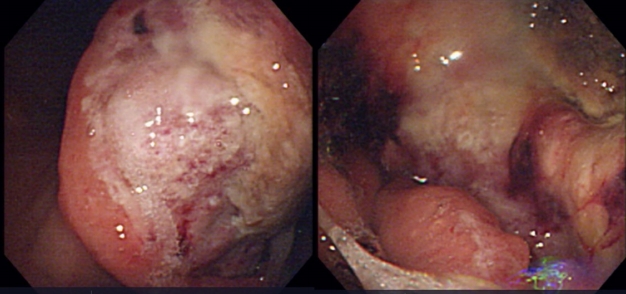
Esophagogastroduodenoscopic findings show a huge ulcerofungating mass involving the antrum and the lower body of the stomach, leading to obstruction of the pyloric ring.
Two years later (2005), he developed gross hematuria, and an intravenous pyelogram showed irregular intraluminal filling of the bladder, suggesting bladder cancer (Fig. 2). Transitional cell carcinoma was diagnosed by pathology. He underwent transurethral resection of the bladder more than four times.
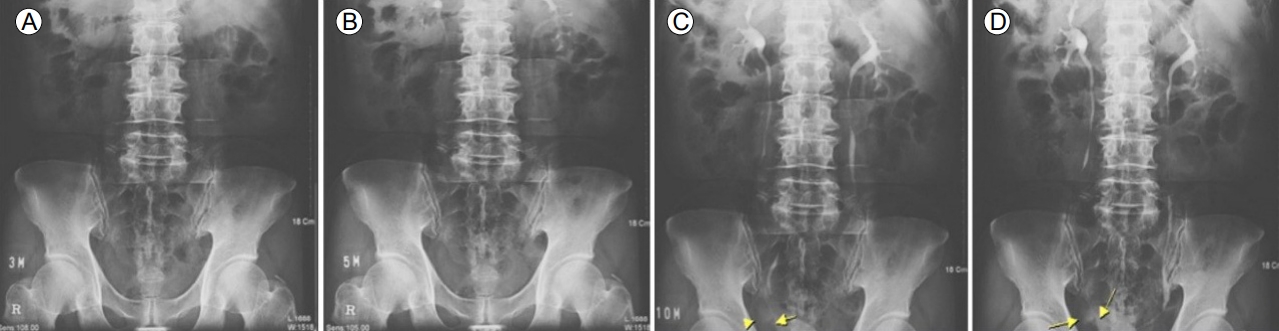
Intravenous pyelogram showing irregular intraluminal filling in the right lateral portion of the bladder (arrows and arrowhead), suggesting bladder cancer. (A) Nephrogram phase (3 minutes film). (B) Secretory phase (5 minutes film). (C) Pyelographic phase (10 minutes film). (D) Pyelographic phase (13 minutes film).
Five years later (2010), he presented with swallowing difficulty of more than 2 months’ duration. Follow-up neck magnetic resonance imaging revealed a 1.3-cm soft-tissue mass in the retropharyngeal space at the level of the epiglottis (Fig. 3). The clinical diagnosis was hypopharyngeal squamous cell carcinoma. Partial pharyngectomy was performed followed by postoperative radiation therapy.

Neck magnetic resonance image shows a 1.3-cm soft-tissue mass in the retropharyngeal space at the level of the epiglottis. On the T1-weighted image, intense enhancement (arrow) was compatible with vascular structures.
Three years later (2013), he developed dizziness and dyspnea. Computed tomography (CT) showed a 2.3-cm endoluminal mass in the right renal pelvis, suggesting urothelial cancer (Fig. 4). He underwent right nephroureterectomy.
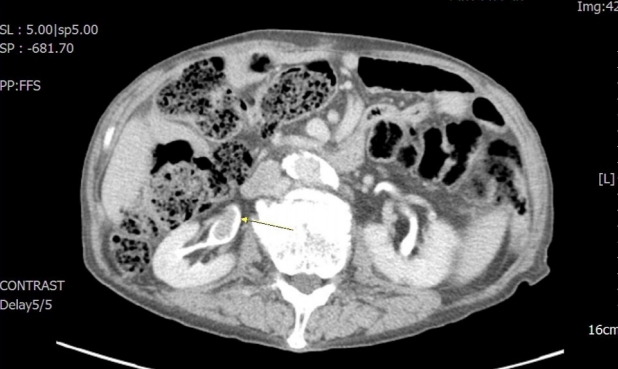
On enhanced abdominal computed tomography images, a 2.3-cm endoluminal mass (arrow) is seen in the right renal pelvis.
The next year (2014), during regular follow-up, an abdominal ultrasound showed a 1.8-cm low-echoic lesion in the right lobe of the liver. CT of the liver showed an increased area (3.5 cm) of fat that contained a low-attenuating mass in hepatic segment V (Fig. 5). The tumor was histologically confirmed as hepatocellular carcinoma (HCC). After one session of radiofrequency ablation, a multidetector CT exam of the liver showed an enhancing soft-tissue lesion in the lower esophagus, later confirmed by pathology as squamous cell carcinoma with possible metastatic deposition in the gastrointestinal tract. The patient was treated only with palliative therapy.
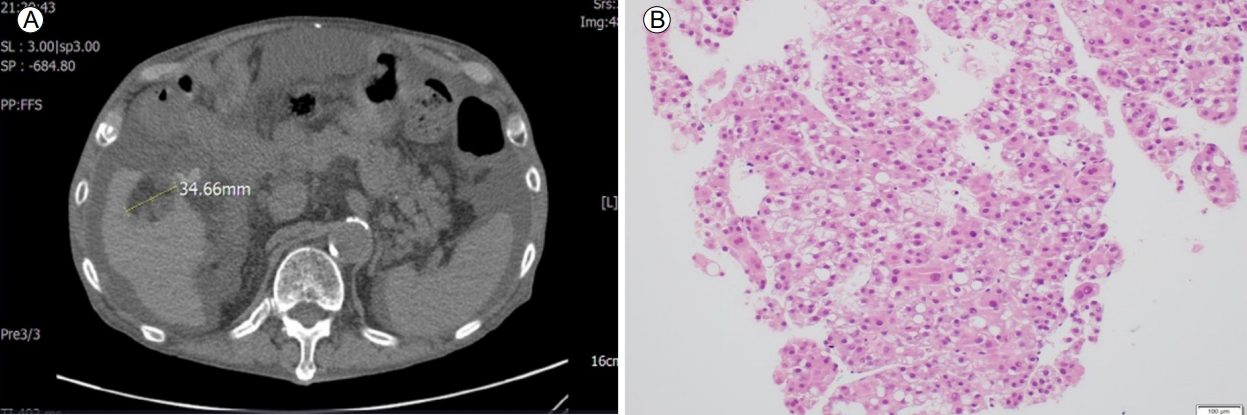
On non-enhanced liver computed tomography images, a 3.5-cm area of fat containing a low-attenuating mass is present in segment V of the liver (A). Histopathological study of the liver specimen shows poorly differentiated malignant cells, consistent with hepatocellular carcinoma (B) (H&E stain, ×200).
In the 3 years since the diagnosis of five different primary cancers, the patient has been lost to follow up. The final diagnosis was multiple, metachronous primary malignancies, probably secondary to smoking.
DISCUSSION
The following three criteria define MPMs: 1) Each tumor must be distinct from the others. 2) The tumors must present with definite features of malignancy. 3) The possibility that one tumor is a metastasis of another must be ruled out [12,13]. The prevalence of MPMs has increased, and in western countries 11.0-21.0% of all cancers involve more than one primary [14]. Case reports and studies with small sample sizes have investigated MPMs, including HCC [15,16]. In 2002, 11.5 % of all MPMs in Korea included liver cancer [17].
Several environmental factors, such as immunity, heredity, nutrition, and chemotherapeutic regimens, are thought to play a role in the development of primary and second malignancies [18]. In our patient, the multiple malignancies were confirmed to be separate entities, although they shared several etiologic factors, such as smoking. Our patient was a smoker, which is a well-documented risk factor not only for gastric, bladder, and urothelial cancer, but also for HCC. In the series of Kurishima et al. [18], 77.6% of the patients with multiple primary tumors were habitual smokers, including 68 patients (69.4%) with a 30 pack-year or more history of smoking. Fifty-seven (85.1%) of 67 patients with aerogastrointestinal and head and neck cancers were smokers. The development of a second primary is also a well-known consequence of chemotherapy [19,20], but our patient did not receive chemotherapy for any of the primary tumors.
It has been reported that a greater percentage of multiple primary cancers occur in the same organ or in organs of the same system than in unrelated organs [21]. The association between unrelated organs (stomach, bladder, pharynx, and liver) in our patient suggests the circulation of carcinogenic metabolites. Environmental and metabolic factors also played a role, including an unhealthy lifestyle, diabetes, and hypertension. The finding of Kurishima et al. [18], that most of the first primary tumors occurred in the gastrointestinal tract, was consistent with the pattern in our patient.
MPMs are challenging for both clinicians and patients because the treatment options are usually limited. A thorough clinical history is important to the management of MPMs, as it can explain the clinicopathological manifestations and identify the prognostic factors. Large-scale studies are therefore needed.
In conclusion, to the best of our knowledge, this is the first case report of five metachronous malignancies in a Korean patient. Despite the rarity of such cases, clinicians should be aware of the possibility of multiple malignancies during treatment of cancer patients. An aggressive follow-up should be performed to determine the long-term survival of patients with MPMs.