거대 결절성 부신피질 과증식에 기인한 쿠싱증후군 1예
Cushing Syndrome Caused by ACTH-independent Macronodular Adrenal Hyperplasia
Article information
Trans Abstract
Adenocorticotropic hormone-independent macronodular adrenal hyperplasia (AIMAH) is a rare cause of Cushing’s syndrome. It is characterized by multinodular enlargement of the adrenal glands and hypercortisolism. Although bilateral adrenalectomy is the standard therapy, unilateral adrenalectomy is an effective alternative. Here we present a case of a 71-year-old female referred to the Endocrinology Department for further evaluation of bilateral adrenal macronodular hyperplasia. Based on dynamic hormone tests and imaging studies, she was diagnosed with AIMAH. Due to persistent hypercortisolism, she underwent completion contralateral surgery after unilateral adrenalectomy. This case demonstrates that unilateral adrenalectomy should be considered in a patient presenting with obvious symptoms of hypercotisolism and relatively asymmetric adrenal enlargement.
INTRODUCTION
Adenocorticotropic hormone (ACTH)-independent Cushing’s syndrome accounts for 15-20% of endogenous Cushing’s syndrome cases, as the majority are due to unilateral adrenal adenoma (and, less frequently, to adrenal carcinoma) [1]. ACTH-independent macronodular adrenal hyperplasia (AIMAH), characterized by massive bilateral macronodular enlargement of the adrenal glands and hypercortisolism, is a very rare cause of Cushing’s syndrome.
Bilateral adrenalectomy with subsequent steroid replacement is the standard therapy for AIMAH [2]. However, unilateral adrenalectomy is regarded as a safe alternative for long-term remission in select patients. Here, we present a case of a patient with AIMAH who underwent unilateral laparoscopic adrenalectomy followed by completion remnant adrenalectomy because of persistent hypercortisolism
CASE REPORT
A 71-year-old female presented with dyspnea of 2 months duration prior to her admission. A chest/abdomen computed tomography (CT) scan revealed a pulmonary thromboembolism in the right segmental artery and multiple nodular thickenings in both adrenal glands. She was started on anticoagulation therapy with warfarin. After the dyspnea had improved, she was referred to the Endocrinology Department for evaluation of the multiple nodular thickenings. She had experienced weight gain and developed a puffy face over the previous 6 years, with symptoms increasing progressively during the 2 years prior to admission. Seven years earlier, she had been diagnosed with unstable angina requiring balloon angioplasty with stent placement. A meningioma involving the right cavernous sinus, but excluding the pituitary gland, diagnosed 1 year previously had been treated with gamma-knife radiosurgery.
The abdominal CT scan revealed bilateral adrenal multiple nodular thickening of 2.8 cm and 5.2 cm in the right and left glands, respectively (Fig. 1). She had a resting blood pressure of 142/98 mmHg while on atenolol (25 mg/day) and furosemide (20 mg/day). She also presented with facial plethora, bruises on her arms and legs, truncal obesity, and decreased strength involving the lower limbs that had progressed gradually for 4 months. Her 24-h-urine free cortisol (UFC) level was elevated, at 518.54 μg/day (normal range, 58-403 μg/day), and low- and high-dose dexamethasone suppression tests (2-day method) demonstrated persistently high cortisol levels (Table 1). Basal ACTH was suppressed at 5.12 pg/mL (normal range, 10-60). Serum levels of metanephrine (0.28 mg/day) and vanillylmandelic acid (3.64 mg/day) were in the normal range (< 0.80 and 1.20-6.52, respectively). The plasma aldosterone concentration was 48.5 pg/dL and plasma renin activity was 9.16 pg/mL/h. Thus, the aldosterone/renin activity ratio was 5.29, ruling out primary aldosteronism. Her serum biochemical assay findings were as follows: HbA1c, 6.4%; low-density lipoprotein-cholesterol, 206 mg/dL; triglyceride, 134 mg/dL; high-density lipoprotein-cholesterol, 48 mg/dL; total cholesterol, 270 mg/dL; blood urea nitrogen, 13.2 mg/dL; creatinine, 0.77 mg/dL; sodium, 142 mEq/L; and potassium, 3.8 mEq/L. To exclude pituitary macroadenoma and ectopic ACTH tumors, pituitary magnetic resonance imaging (MRI) and an octerotide scan were performed. As shown in Figures 2 and 3, the dynamic MRI revealed no pituitary tumor, and there was no abnormal focal tracer uptake in the octerotide scan, except in the right cavernous sinus, consistent with the previously diagnosed meningioma. The patient was therefore clinically diagnosed with Cushing’s syndrome with ACTH-independent bilateral adrenal hyperplasia.
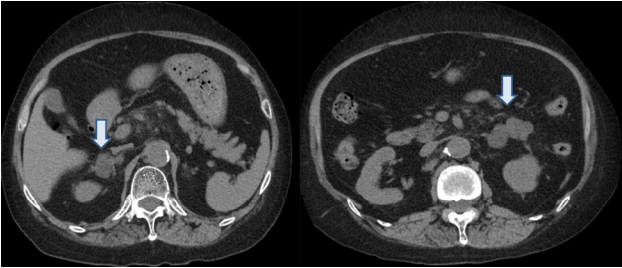
Abdominal computed tomography (CT). Non-contrast-enhanced abdominal CT scan showing bilateral multinodular adrenal enlargement. The nodules had a maximum diameter of 2.8 cm on the right side and 5.2 cm on the left side (arrows).
Pituitary dynamic magnetic resonance imaging (MRI). Dynamic MRI showing a mass in the right cavernous sinus with no involvement of the pituitary gland.

111In-octreotide scan 4 and 24 h after injection. The scan revealed no abnormal focal tracer uptake except in the right cavernous sinus, indicating the previously diagnosed meningioma.
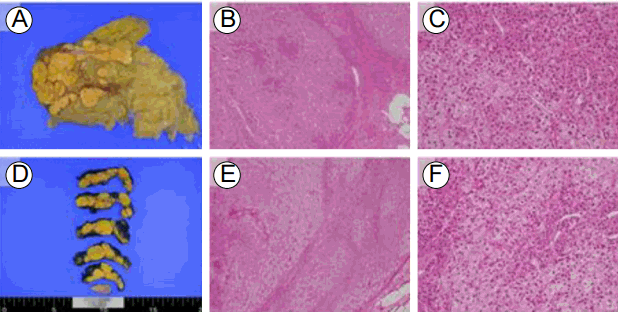
She was initially treated with a unilateral laparoscopic adrenalectomy of the left adrenal gland because of the high surgical risk posed by her age and history of unstable angina and meningioma. The resected left adrenal gland was enlarged, measuring 8.0 × 5.5 × 4.5 cm. Multiple golden-yellow, unencapsulated nodules of various size were observed on the cut section (Fig. 4A). Microscopic examination showed nodules composed predominantly of large and clear cells and a few small compact cells with variable amounts of lipid (Fig. 4B and 4C). Mitotic figures and cellular pleomorphism were absent. One month after surgery, the patient had persistent and severe symptoms of hypercortisolism, including proximal limb weakness and a swollen face. Her 24-h UFC was 1,046.72 μg/day, higher than previously determined. She therefore underwent completion contralateral adrenalectomy. The macroscopic and microscopic findings of the right adrenal gland were similar to those of the pre-resected left adrenal gland. The right adrenal gland measured 5.0 × 5.0 × 1.0 cm. Transverse sections showed multiple nodules (Fig. 4D) and a compressed normal gland at the peripheral portion. The nodules were also composed of lipid-rich clear cells and lipid-depleted compact cells (Fig. 4E and 4F). Pathological analysis revealed nodular adrenal cortical hyperplasia.

Macroscopic and microscopic findings of the left and right adrenal glands. Both adrenal glands are enlarged and contain multiple nodules, as seen on the cut section (A: left, D: right). The unencapsulated nodules are composed of lipid-rich pale cells and lipid-depleted eosinophilic cells (B: left, E: right; hematoxylin and eosin staining, ×4) A high-power view shows large, clear vacuolated cells and small compact cells (C: left, F: right; hematoxylin and eosin staining, × 20).
Complete adrenalectomy resolved the patient’s Cushing’s syndrome, as both her 24-h UFC levels and serum ACTH levels were within normal ranges during follow-up (179.84 μg/day and 23.12 pg/mL, respectively). She was started on replacement hydrocortisone, which was tapered to 15 mg/day. Her plasma ACTH and cortisol levels remained stable postoperatively. Despite stabilization of her disease symptoms, the patient died from underlying heart disease 11 months after adrenalectomy.
DISCUSSION
This report describes a patient with Cushing’s syndrome related to AIMAH, a benign tumor and rare cause of Cushing’s syndrome [3]. The incidence of AIMAH is equally distributed between sexes and peaks in the fifth and sixth decades of life, which is later age of onset than that of unilateral adenoma [2].
The pathophysiology of AIMAH is heterogeneous. Rare mutations in several genes, including those encoding Gs alpha, melanocortin receptor type 2, phosphodiesterase 11A, and fumarate hydratase, have been reported [4]. Several studies have shown that AIMAH is regulated by hormones other than ACTH through aberrant expression of membrane-bound receptors in the adrenocortical nodular glands [5]. The characteristic hyperplasia can be explained by the aberrant stimulation of gastric inhibitory peptide, catecholamine, beta-adrenergic receptors, vasopressin, serotonin, luteinizing hormone, and human chorionic gonadotropin receptor, which also may be responsible for cortisol hypersecretion [5]. A recent pathophysiological study showed that cortisol production was regulated by aberrant membrane receptors and by corticotropin produced within the adrenocortical tissue. Thus, “bilateral macronodular adrenal hyperplasia with ectopic expression of corticotropin within the adrenal cortex” would be more accurate description of this disease [6].
Bilateral adrenalectomy is the standard treatment for AIMAH, but these patients subsequently require lifetime steroid replacement and are susceptible to adrenal insufficiency. For these reasons, unilateral adrenalectomy was proposed recently as a therapeutic alternative in patients with AIMAH. In one study, Cushing’s syndrome was resolved in almost all patients who underwent unilateral adrenalectomy, as indicated by the normalization of their UFC and ACTH levels [3] and amelioration of the physical symptoms of Cushing’s syndrome. Because of our patient’s poor performance status, and due to her advanced age and underlying cardiopulmonary disease, treatment initially consisted of unilateral adrenalectomy. However, aggravation of her hypercortisolism, evidenced by her deteriorating health and increased 24-h UFC, required complete contralateral adrenalectomy. Her daily UFC and ACTH levels then normalized.
Previous reports demonstrated that unilateral adrenalectomy is effective in older patients with a high operative risk [7]. However, it is successful mainly in patients with asymmetric adrenal enlargement or with mild hypercortisolism [3]. In our patient, the adrenal enlargement was relatively asymmetric and cortisol secretion was relatively mild. We did not perform selective adrenal venous sampling or measure cortisol content per gram of tissue in either adrenal lesion. However, we speculated that cortisol production by the resected adrenal gland was much lower than that of the gland remaining after the first operation, despite the difference in size of the two glands. These clinical manifestations justified complete removal of both adrenal glands. A similar case involving a 39-year-old AIMAH patient was reported in 2009 in Korea. Unilateral adrenalectomy was performed initially based on the results of adrenal venous sampling [8], but completion adrenalectomy was deemed necessary because of an elevated 24-h UFC and enlargement of the remnant adrenal gland 2 years after the first operation [9].
To avoid lifelong steroid replacement, medical treatment with specific antagonist or agonist agents may be an alternative to bilateral adrenalectomy. Screening protocols have been developed to identify aberrant adrenal hormone receptors in AIMAH patients [10]. The tests are performed over a period of 3 days and involve modulating the plasma levels of diverse hormone ligands for the suspected aberrant receptors, while monitoring plasma levels of cortisol, other steroids, and ACTH. A patient with positive response to the screening protocol may respond to specific pharmacological therapies as an alternative to adrenalectomy. Since the aberrant expression of membrane-bound receptors is an important pathophysiological component of AIMAH, the failure to perform a screening test was a limitation of our study. Nonetheless, our results demonstrate that unilateral adrenalectomy should be considered in a patient presenting with obvious symptoms of hypercotisolism and adrenal enlargement that is relatively asymmetric.
In summary, A 71-year-old patient diagnosed with ACTH-independent Cushing’s syndrome required completion contralateral surgery after unilateral adrenalectomy because of severe hypercortisolism. Since the success or failure of unilateral adrenalectomy depends on the individual characteristics of the patient and the adrenal tumor, AIMAH must be treated carefully on a case-by-case basis.
