위십이지장동맥 동맥류와 십이지장 사이 누공에서의 만성 출혈
Chronic Hemorrhage from a Fistula between a Gastroduodenal Artery Aneurysm and the Duodenum
Article information
Trans Abstract
Gastroduodenal artery (GDA) aneurysm is a very rare event, but it induces life-threatening clinical manifestations upon rupture or bleeding. The causes of GDA aneurysm are atherosclerosis, infection, trauma, surgery, iatrogenic lesions, mycotic or tuberculous disease, and autoimmune disease. We report the case of a 77-year-old female who presented with melena and vomiting. Upper gastrointestinal endoscopy revealed a 4 cm pulsatile extrinsic mass with a duodenal fistula at the duodenal bulb. Abdominal computed tomography showed a large aneurysm of the GDA. We successfully conducted transcatheter embolization of the aneurysm. After the procedure, the patient did not present with melena, and her hemoglobin level was stable. Follow-up endoscopy showed that the GDA aneurysm-duodenal fistula had decreased in size and was leaking a small amount of blood. An arteriography was performed and showed no evidence of contrast filling in the aneurysmal sac.
INTRODUCTION
Gastroduodenal artery (GDA) aneurysm is a rare condition, but it can induce very serious conditions if rupture occurs [1]. The causes of GDA aneurysm are degeneration, atherosclerosis, fibromuscular dysplasia, and collagen disorders [2]. GDA aneurysm accounts for 0.5-1.5% of all visceral aneurysms. Chronic pancreatitis is the most common clinical manifestation associated with GDA aneurysm; other conditions include liver cirrhosis, trauma, septic emboli, and vascular abnormalities. Among the diagnostic devices for GDA aneurysm, angiography has the highest sensitivity [1]. Management of GDA aneurysm includes endovascular transcatheter embolization and surgical therapy; the former is the most common method and was used in this case. We report our experience with treatment of a bleeding patient who had a fistula between a GDA aneurysm and the duodenum.
CASE REPORT
A 77-year-old female was admitted to our hospital with melena and vomiting for 2 h. She had presented with intermittent melena 3 weeks prior to this admission. She had undergone partial thyroidectomy because of a benign thyroid nodule 30 years ago. The patient’s blood pressure was 90/60 mmHg, and her pulse rate was 90/min. Digital rectal examination was positive for melena, and old clots were drained using a Levin tube. Laboratory tests showed a hemoglobin level of 6.5 g/dL, iron level of 43 µg/dL, total iron binding capacity of 301 µg/dL, unsaturated iron binding capacity of 258 µg/dL, and ferritin level of 31 ng/mL. During upper gastrointestinal endoscopy (EGD), a 10 mm ulcerative mucosal lesion was found on the anterior wall of the distal bulb. The ulcerative lesion was on an extrinsically compressive mass 4 cm in size (Fig. 1A). The extrinsic mass was pulsating, suggesting that it might be an arterial structure; thus, we did not attempt endoscopic treatment of the ulcer. Arterial-phase abdominal computed tomography (CT) showed a 5.8 × 5.8 cm aneurysm originating from the GDA compressing the duodenal bulb (Fig. 1B and 1C). Therefore, we assumed that the ulcerative lesion was a fistula between the GDA and duodenal bulb. There was a variation in hepatic artery anatomy; the right hepatic artery originated from the posterior pancreaticoduodenal artery (PPDA).
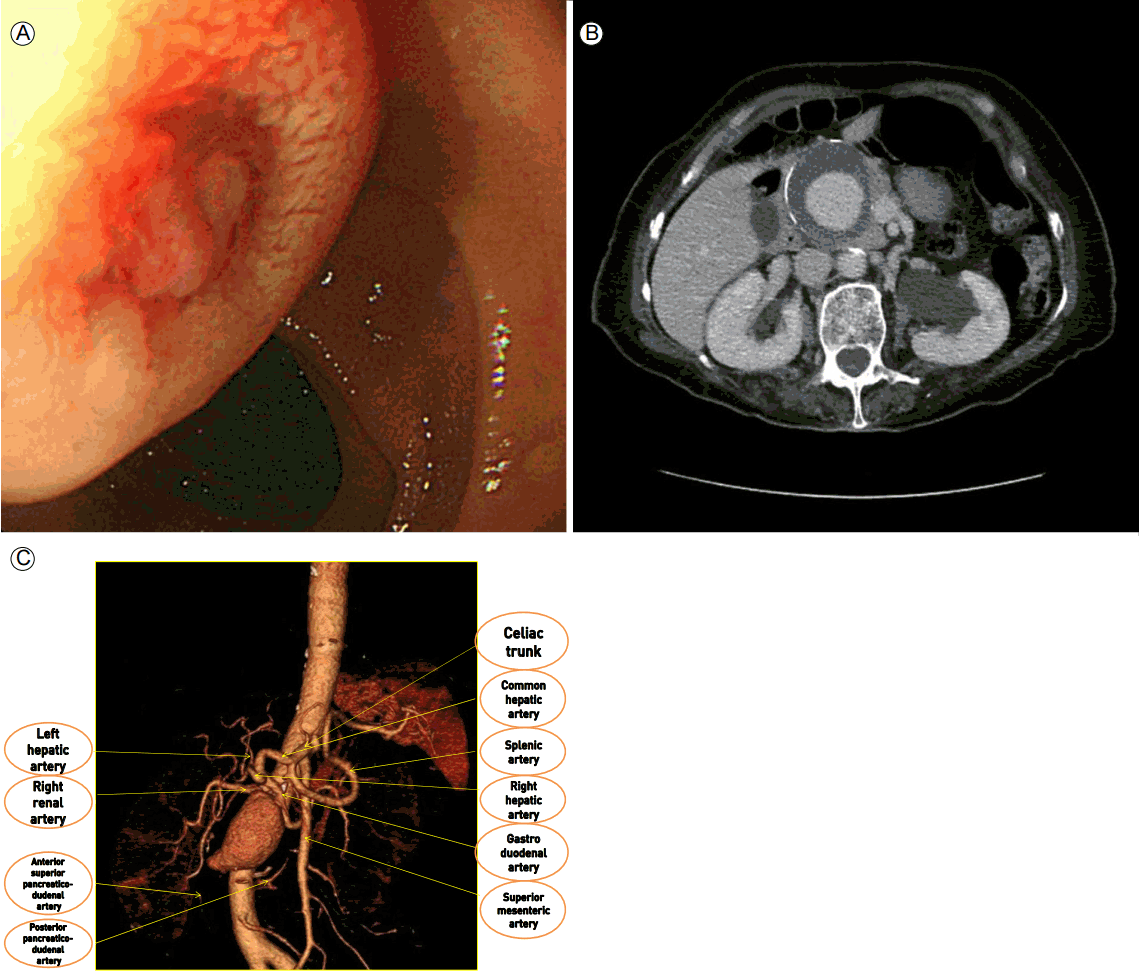
Endoscopic findings. (A) A pulsating mass with a fistula on the anterior wall of the duodenal bulb. An axial arterial-phase abdominal computed tomography scan (B) shows a large, partially thrombosed aneurysm in the upper abdomen. Volume-rendered image (C) shows that the aneurysm originates from the gastroduodenal artery.
The patient was referred to the interventional department of radiology for potential endovascular treatment of the aneurysm. Through a right femoral approach, the celiac axis and a common hepatic arteriogram were obtained using a 5 Fr RH catheter (Cook Medical, Bloomington, IN, USA). The arteriogram revealed a large aneurysm of the GDA (Fig. 2A). The anterior superior pancreaticoduodenal artery (ASPDA) arose just distal to the neck of the aneurysm. Contrast extravasation from the aneurysm into the duodenal lumen suggested that active bleeding was not present. The left hepatic artery alone arose from the common hepatic artery. We speculated that the flow of the right hepatic artery was from the superior mesenteric artery (SMA) through the PPDA.

Common hepatic arteriogram (A) shows a large aneurysm of the gastroduodenal artery. Celiac axis arteriogram (B) obtained after embolization shows complete occlusion of common hepatic arterial flow. There are multiple coils within the aneurysmal sac, which migrated during the procedure.
Embolization of the aneurysm was performed. First, the ASPDA distal to the aneurismal neck was embolized using three 0.018-inch coils (MicroNester; Cook Medical) 4 mm in diameter and 70 mm in length via a 2.0 Fr microcatheter (Progreat, Trumo Co., Tokyo, Japan). Then, embolization of the GDA proximal to the aneurismal neck was attempted using multiple 0.035-inch coils (Nester; Cook Medical) 8-16 mm in diameter and 14 mm in length via a 5 Fr RH catheter. However, some of the coils migrated into the sac due to high-pressure flow into the sac. Finally, we embolized the common hepatic artery including the short segment of the proximal left hepatic artery and the proximal neck of the aneurysm. A completion angiogram revealed successfully occluded flow into the aneurysmal sac (Fig. 2B). On SMA arteriography, flow into the right hepatic artery via the PPDA was preserved.
After endovascular treatment and red blood cell transfusion, the patient’s hemoglobin level was maintained at ~10 g/dL for 3 days, and she did not present with melena. The patient did not undergo any medical treatments, such as an intravenous proton pump inhibitor or tranexamic acid. Abdominal CT obtained 3 days later revealed persistent contrast filling within the aneurysmal sac, suggesting the absence of flow into the sac (Fig. 3). No ischemic injury to visceral organs was noted. Follow-up endoscopic examination on post-procedural day 8 revealed that the fistula and pulsating mass had decreased in size; however, a small amount of blood was leaking from the fistula in time with peristalsis (Fig. 4A). On post-procedural day 15, the patient underwent follow-up EGD. The size of the pulsating mass and fistula was decreased compared with that on post-procedural day 8. However, the blood leakage persisted. We attempted to close the fistula by clipping, but this failed. Her vital signs were stable, and her hemoglobin level was not decreased at 15 days after the endovascular procedure. Moreover, there was no communication between the GDA and aneurismal sac, which was confirmed by follow-up angiography. We concluded that the bleeding had stopped, and the blood observed in the duodenal lumen on post-procedural day 15 was stagnating from a prior leakage. On post-procedural day 25, the patient underwent follow-up angiography to assess vascular flow to the aneurismal sac. We did not find any evidence of vascular communication or contrast leakage into the aneurysmal sac. On post-procedural day 83, follow-up EGD was performed. The size of the fistula decreased to a pin-point, and no blood leakage was found (Fig. 4B). On the same day, her hemoglobin level was 10.8 g/dL. Subsequently, the patient did not experience specific symptoms and underwent a screening EGD 8 months after the procedure. During this EGD, several coils were noted in the duodenal bulb at the fistula that exited the aneurismal sac through the fistulous tract (Fig. 4C).

Axial precontrast abdominal computed tomography, obtained 3 days after embolization, shows remaining contrast within the aneurysmal sac, suggesting the absence of flow into the sac.

Follow-up endoscopic findings. (A) The fistula is smaller than seen on the previous image, but it oozes blood intermittently. (B) The fistula decreased to a pin-point size, and no blood leakage was found. (C) Extraction coil through the fistulous tract between the aneurysm and duodenum was observed in the duodenal bulb.
DISCUSSION
In the present case, a GDA fistula was the cause of chronic gastrointestinal bleeding. Although the fistula developed between the GDA and duodenal bulb wall, massive bleeding did not occur. Gradual bleeding without hemodynamic derangement via an arterial aneurysm is an uncommon event; thus, we were uncertain that the ulcerative lesion was a fistula between the GDA and duodenal bulb, or that the fistula was the cause of gastrointestinal bleeding. However, this was confirmed by the findings that after treatment, the hemoglobin level was maintained without oral iron supplementation for 8 months, and the coils exited the aneurismal sac through the fistula.
A fistula can be mistaken for an ulcer on EGD. However, in the current case, the shape of the lesion differed from that of an ulcer in that it was dented and located at the apex of the extrinsic compressive mass. This revealed a slowly oozing bleeder in the bulb of the duodenum associated with a protruding submucosal pulsation into the duodenal wall.
The most common cause of aneurysm is atherosclerosis, followed by pancreatitis, infection, blunt trauma to the upper abdomen, autoimmune disease, and vascular intervention and surgery [3,4]. The patient in this case had none of these risk factors and no medical history.
Gastrointestinal bleeding from a GDA aneurysm is a very rare event, comprising only 1.5% of all reported peripheral aneurysms. In this case, chronic hemorrhage occurred instead of massive bleeding through the fistula between the GDA aneurysm and duodenum. On EGD, blood leakage was observed with peristalsis but not with pulsation. This might be because the fistula formed chronically, and some barrier in the fistula could stand the arterial. However, this hypothesis could not be evaluated by histology.
Visceral angiography is the gold standard diagnostic test for GDA aneurysms [5]. Therapeutic devices use either an endovascular transcatheter or surgical approach. The goal of any procedure is to prevent aneurysm dilation and rupture [6]. Endovascular transcatheter embolization is the most common method, but it has a risk of visceral ischemia and organic infarction [7]. Endovascular transcatheter embolization is the treatment of choice for hemodynamically stable patients, whereas surgical therapy is needed for patients with previously unsuccessful embolization or a hemodynamically emergent condition caused by active bleeding [8]. Endovascular transcatheter embolization is a more effective alternative to surgical therapy for the management of visceral artery aneurysms [7]. If the diagnosis of GDA aneurysm is made before rupture, and the condition is managed properly, the prognosis is relatively good [1].
Habib et al. [9] reported a case of, and proposed a treatment algorithm for, GDA aneurysms. For ruptured GDA aneurysms, open surgery is recommended if the patient is hemodynamically unstable. Endovascular repair (coil embolization or stent placement) is the treatment of choice for non-ruptured or ruptured GDA aneurysms in hemodynamically stable patients.
The present case shows that coil occlusion of the GDA was successful in treating chronic hemorrhage through a fistula between the GDA aneurysm and duodenum. We thus prevented an emergency event due to melena and chronic anemia resulting from fistula formation. If diagnosis and radiologic management were delayed, the patient might have died due to aneurysmal rupture and massive gastrointestinal bleeding.
GDA aneurysm is a very unusual event that can be fatal upon rupture or bleeding. We report herein the case of a 77-year-old female who complained of melena and vomiting. Upper gastrointestinal endoscopy and abdominal CT were used to detect a GDA aneurysm with a duodenal fistula at the duodenal bulb. Therefore, we performed transcatheter embolization of the aneurysmal sac. The procedure was successful, after which the patient was in a stable condition.
Endovascular transcatheter angiography is the gold standard diagnostic tool for GDA aneurysms that can also be used for their treatment. This case shows that coil embolization of the GDA aneurysm was successful in treating chronic hemorrhage through a fistula between the GDA aneurysm and duodenum. Therefore, we prevented an emergency event caused by melena and chronic anemia.