타카야수 혈관염 환자에서 발생한 관상동맥-쇄골하동맥 도류증후군
Coronary-subclavian Steal Syndrome in a Patient with Takayasu Arteritis
Article information
Trans Abstract
A 37-year-old woman who had undergone coronary artery bypass grafting (CABG) surgery for left main and right coronary ostial lesions 2 years prior presented with angina and transient visual dimness. Computed tomography angiography showed a patent left internal mammary artery (LIMA) bypass graft and concentric narrowing with perivascular thickening around the arch vessels. The patient was diagnosed with Takayasu arteritis with coronary subclavian steal syndrome (CSSS). Thoracic angiography revealed severe stenosis of the left proximal subclavian artery (SCA) and reverse flow from the coronary artery to the distal left SCA via the LIMA graft. Successful percutaneous stenting of the left SCA was performed together with stenting of the right common carotid artery (CCA). The patient’s symptoms were completely resolved. This case is informative since it shows that Takayasu arteritis can manifest as angina due to coronary ostial lesions and then can involve arch vessels, which can lead to CSSS in patients with CABG.
INTRODUCTION
Coronary subclavian steal syndrome (CSSS) is a rare complication of coronary artery bypass graft (CABG) surgery with a left internal mammary artery (LIMA) graft. It is defined as reverse blood flow from the coronary artery to the distal subclavian artery (SCA) via the LIMA that results in myocardial ischemia. CSSS most commonly results from obstructive atherosclerotic disease. We present a case of CSSS due to inflammatory stenosis of the left SCA in a woman with Takayasu arteritis (TA) who was treated with percutaneous angioplasty of the left SCA.
CASE REPORT
A 37-year-old woman underwent off-pump CABG using an in situ LIMA graft to the left anterior descending (LAD) coronary artery, and a free right internal mammary artery (RIMA) graft from the LIMA to the obtuse marginal branch of the left circumflex (LCx) coronary artery to treat unstable angina. Preoperative coronary angiography revealed severe ostial stenosis of the left main coronary artery and 50% ostial stenosis of the right coronary artery. Preoperative ultrasonography showed no stenosis of the common carotid artery (CCA) and SCA with forward flow through both internal mammary arteries (Fig. 1A-1D). C-reactive protein (CRP) was 2.2 mg/dL at that time. The difference in systolic blood pressure (BP) in both arms was less than 10 mmHg.
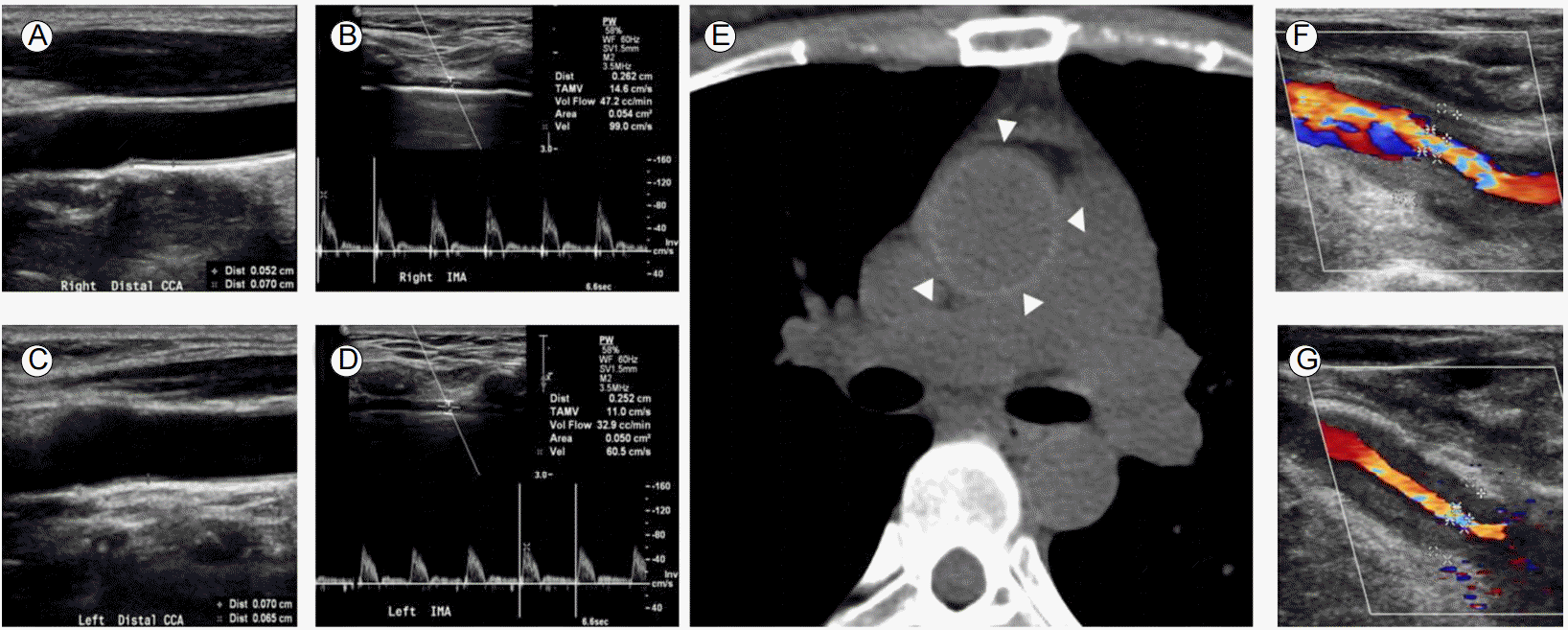
Progression of vascular lesions in the aortic arch branches. (A, C) Preoperative duplex ultrasonography revealing no stenosis in both common carotid arteries. Intimomedial thickness was 0.052 cm and 0.070 cm in the right and left carotid arteries, respectively. (B, D) Doppler examination of both internal mammary arteries demonstrated forward flow. (E) Preoperative non-contrast CT of the aorta showing a highly attenuating ring in the ascending aorta (triangles). (F, G) Two years after CABG, more than 70% stenosis was noted in the right and left carotid arteries. CT, computed tomography; CABG, coronary artery bypass graft.
After 2 years, the patient presented with chest pain upon exertion and transient visual dimness. BP measurements were 85/67 mmHg and 61/50 mmHg in the right and left arms, respectively. Ultrasonography revealed diffuse concentric narrowing of both CCAs with increased intimomedial wall thickness (Fig. 1F and 1G). Coronary computed tomography (CT) angiography revealed a patent LIMA-LAD artery graft (Fig. 2A). Diffuse enhanced wall thickening around the left SCA, bilateral CCAs, right innominate artery, aortic root, ascending aorta, and aortic arch with concentric narrowing of the left SCA, bilateral CCAs, and right innominate artery were also observed (Fig. 2B and 2C). Brain magnetic resonance (MR) perfusion imaging showed mild perfusion delay in the territory of the left middle cerebral artery and left posterior inferior cerebellar artery (Fig. 2D). TA was diagnosed based on fulfilling 4/6 of the criteria from the American College of Rheumatology; age less than 40 years, limb claudication, difference in systolic BP greater than 10 mmHg, and arteriogram abnormality. Retrospective review of non-contrast CT of the ascending aorta taken before CABG surgery showed a concentric highly attenuated ring from the aortic root to the ascending aorta (Fig. 1E), which implies that ostial stenosis of the coronary arteries was due to TA. CRP was 4.9 mg/dL. Because of the active status of her TA, prednisolone 1 mg/kg/day was started and she was discharged. Left subclavian revascularization was scheduled after control of acute inflammatory state.

Vascular imaging and percutaneous transluminal angioplasty after presentation with angina and visual dimness. (A) Three-dimensional volume-rendered image showing severe stenosis of the left subclavian artery (SCA) proximal to the origin of the left internal mammary artery (LIMA) and patent LIMA graft from the SCA to the left anterior descending (LAD) coronary artery. (B) CT angiography showing concentric wall thickening with luminal stenosis in the right innominate artery, left common carotid artery (CCA) and left SCA. Mural enhancement and low attenuation of the inner concentric ring indicate an active stage of Takayasu arteritis. (C) CT angiography demonstrating concentric wall thickening of the aortic root (triangles), moderate ostial stenosis of the right coronary artery (open arrow), and total occlusion of the left main coronary artery (closed arrow). (D) Magnetic resonance perfusion imaging of the brain demonstrates mild perfusion delay in the territory of the left middle cerebral artery and left posterior inferior cerebellar artery. (E) Thoracic aortogram revealing stenosis in the right brachiocephalic artery, severe stenosis of the right CCA, long-segmental severe stenosis of the left CCA, and severe stenosis of the left SCA. The LIMA appears distal to stenosis of the left SCA. (F) Successful balloon angioplasty and stent deployment were performed at the left proximal SCA together with stenting from the right innominate artery to the right proximal CCA. Selective angiogram revealing patent left SCA and LIMA (G) and delayed phase showing antegrade flow through the LIMA bypassed to the LAD and right internal mammary artery (RIMA) connecting the LIMA and the obtuse marginal branch of the left circumflex artery (H). CT, computed tomography; OM, obtuse marginal artery; LCx, left circumflex artery.
However, on the following day, the patient presented to the emergency department with acute chest pain. Electrocardiogram showed sinus rhythm with no ST or T wave changes. Troponin I was increased to 12.32 ng/mL (normal range 0.00-0.78 ng/mL). The patient was anticoagulated with unfractionated heparin for management of non-ST elevation myocardial infarction (NSTEMI). Immediate thoracic aortography confirmed severe stenosis involving major vessels arising from the aortic arch (Fig. 2E). Successful balloon angioplasty with stent deployment of the left proximal SCA was performed together with stenting from the right innominate artery to the right proximal CCA (Fig. 2F). Selective angiography of the left SCA demonstrated patent antegrade LIMA flow to the LAD and LIMA-RIMA-LCx flow (Fig. 2G and 2H). Following the intervention, angina and transient visual dimness were completely resolved. Follow-up BP measurements showed 74/46 and 95/47 mmHg in the right and left arms, respectively.
DISCUSSION
In this study, we described a case of TA presenting with CSSS caused by inflammatory stenosis of the left SCA not related with atherosclerotic disease. TA is a nonspecific inflammatory process of unknown etiology that involves the aorta and its main branches [1,2]. Coronary arterial involvement occurs in 10-30% of patients with TA [3]. However it is rare for TA patients to present with coronary ostial stenosis without the involvement of other arch vessels [4]. In this case, it is interesting that the coronary arteries were the first site of vascular obstruction by TA, which was then followed by de novo severe stenotic lesions in the arch vessels found 2 years later (Fig. 3). Another unusual feature is that although the onset age for TA is typically between 10 and 20 years [3], this patient’s active vasculitis first manifested in her 30s. Recurrent angina after successful CABG related to CSSS most often results from obstructive atherosclerotic disease in the proximal SCA [5], although a few other conditions do lead to CSSS. Only two cases of CSSS in TA patients have previously been reported [6,7].
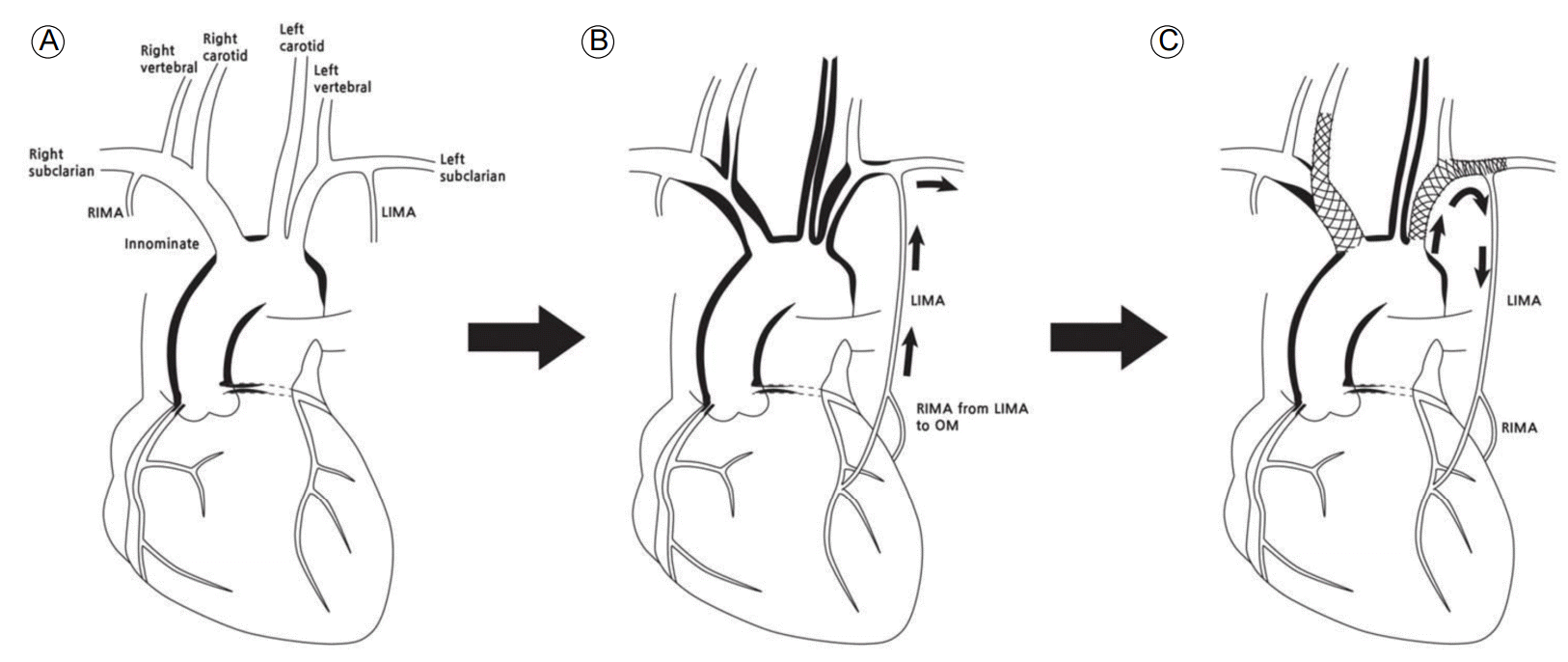
Schematic diagram showing progression and intervention of the vascular lesions in the aortic arch branches of the patient. (A) At initial presentation, vascular lesion involved the coronary ostia and aorta without involvement of the cervical neck vessels. (B) As the disease progressed, vasculitic stenosis involved proximal common carotid arteries, the right innominate artery, and the left subclavian artery (SCA), resulting in retrograde flow from the left internal mammary artery (LIMA) to the left SCA. (C) After stent deployment, forward flow from the left SCA to the coronary artery via the LIMA recovered. In addition, a stent from the right innominate and right common carotid artery was deployed. RIMA, right internal mammary artery; OM, obtuse marginal branch of the circumflex artery.
Doppler ultrasonography for preoperative evaluation revealed no abnormal findings in the arch vessels. In addition, there was no other manifestation of TA shown in the preoperative physical examination. However, coronary ostial stenosis in a young female without atherosclerotic risk factors together with elevated CRP and a highly attenuating ring in the ascending aorta found on non-contrast CT strongly suggests that she had TA before CABG surgery. The most unusual findings in this case were that there was no involvement of the arch vessels at the time of CABG and that there was rapid progression of the inflammatory vasculitic stenosis of the arch vessels for a relatively short period of 2 years.
Successful angioplasty was performed for the left SCA lesions and the stenosis from the right innominate to right CCA to relieve angina and transient visual dimness, respectively. After stent implantation, the patient’s coronary and cerebral ischemia symptoms were completely relieved. Control of TA is important to maintain the patency of revascularized arteries. Immunosuppressive therapy during the peri-procedural period should be considered for better patency of the revascularized vessels in patients with active TA [8]. Hence, angioplasty was scheduled after several weeks of treatment with high-dose steroid therapy. However, NSTEMI forced us to perform angioplasty during the acute phase. Meticulous surveillance of restenosis is needed during the follow-up period.
In conclusion, we report a case of CSSS in a young woman with TA who had undergone CABG surgery. As illustrated by our case, TA should be considered in the differential diagnosis of young female patients with angina, particularly those with coronary ostial lesions [9]. Furthermore, clinicians should keep a vigilant watch for de novo progression of arch vessel stenosis, which could result in CSSS in patients with TA who have undergone CABG surgery.