위 MALT 림프종 완전관해 6개월 후 진단된 대장 MALT 림프종 1예
Colonic MALT Lymphoma Diagnosed 6 Months after Complete Remission of Gastric MALT Lymphoma
Article information
Trans Abstract
Most mucosa-associated lymphoid tissue (MALT) lymphomas are found in the gastrointestinal tract. The most common site is the stomach, whereas colon MALT lymphomas are rare. There are a few reports of simultaneously diagnosed stomach and colon MALT lymphomas. However, diagnosis of primary colonic MALT lymphoma after complete remission of gastric MALT lymphoma is extremely rare. Although the treatment protocol for gastric MALT lymphoma is well established, there is no consensus protocol for colonic MALT lymphoma owing to its rarity. Herein, we report a case of colonic MALT lymphoma incidentally diagnosed 6 months after completion of radiation therapy for gastric MALT lymphoma and treated via polypectomy, with no recurrence for 3 years.
INTRODUCTION
Mucosa-associated lymphoid tissue (MALT) lymphomas are a distinct group of malignant extranodal lymphoepithelial lesions. They were first described by Isaacson and Wright as lymphomas arising from MALT [1]. They can occur in almost any organ exposed to a persistent stimulus, such as chronic infection or an autoimmune condition [2]. The most common site is the stomach, and other gastrointestinal sites include the large and small bowel. Colonic MALT lymphoma is rare; here we report an extremely rare case of a primary colonic MALT lymphoma presenting 6 months after completion of radiation therapy for gastric MALT lymphoma.
CASE REPORT
A 78-year-old man who had experienced post-prandial abdominal discomfort for a few months was admitted to our department. He had no specific past medical or surgical history and no autoimmune disease. His laboratory findings were normal, except for a slightly low hemoglobin level. His vital signs were as follows: blood pressure, 130/70 mmHg; pulse rate, 80 beats/min; respiratory rate, 18 beats/min; and body temperature, 36.6°C. A physical examination revealed no abnormalities and no enlarged lymph nodes. To determine the cause of his abdominal discomfort, gastroduodenoscopy was performed. The results showed an approximately 3 × 3 cm flat depressed lesion in the mid-portion of the lesser curvature of the gastric body (Fig. 1A and 1B). Histological examination of multiple biopsies showed lymphoid cell proliferation in the gastric mucosa, and Giemsa staining for Helicobacter pylori was negative. Immunohistochemistry showed that CD20 and Bcl-1 were strongly expressed.
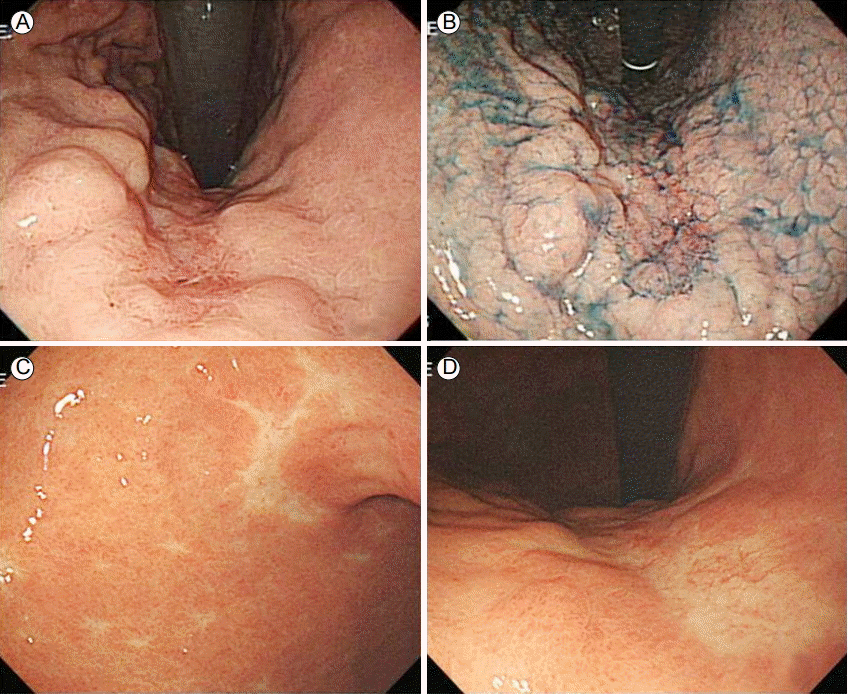
Gastroduodenoscopic findings. (A) A flat depressed lesion in the mid-portion of the lesser curvature of the gastric body is shown. (B) Indigo carmine dye staining of the lesion shown in (A). (C, D) After radiotherapy, a flat discolored scar was observed in the same location as the lesion.
Low-grade gastric MALT lymphoma was diagnosed, and further evaluation was initiated for staging assessment. Abdomen computed tomography (CT) revealed no abnormalities, and positron emission tomography/CT and bone marrow biopsy were not performed. The lymphoma was therefore presumed to be stage IE according to the Ann Arbor classification system. Gastric MALT lymphoma is strongly associated with H. pylori infection; therefore, the patient received a 14-day course of clarithromycin, amoxicillin, and esomeprazole. After treatment completion, gastroduodenoscopy was performed, and multiple biopsies were performed in the gastric antrum and mid-body of the lesser curvature. Unfortunately, the MALT lymphoma was still present in the stomach, and the patient received 17 courses of radiotherapy. Each radiation dose was 180 cGY, and the total dose was 3,060 cGY. Ten weeks after radiotherapy, gastroduodenoscopy and mucosa biopsy showed a complete response. Colonoscopy revealed five tiny colon polyps, which were removed using forceps. Pathologic examination of the polyps revealed tubular adenoma.
Six months after radiotherapy, gastroduodenoscopy revealed a scar at the site of the gastric MALT lymphoma (Fig. 1C and 1D). Giemsa staining for H. pylori was negative. At the same time, colonoscopy for short-term follow-up of the patient’s abdominal discomfort revealed a flat discolored 1.2-cm polyp 20 cm above the anal verge (Fig. 2A). The polyp was removed via polypectomy (Fig. 2B), and the colon was otherwise normal. Pathologic examination of a colonoscopic biopsy revealed lymphoproliferative lesions in the colonic mucosa (Fig. 3A and 3B) that expressed Bcl-2 and CD20 (Fig. 3C and 3D). Primary colonic MALT lymphoma was diagnosed. The resection margin was clear (Fig. 4), and abdomen CT revealed no abnormalities. The lymphoma was classified as stage IE according to the Ann Arbor classification system; therefore no additional treatments were administered, although regular follow-ups were planned. At the 2-year follow-up, gastroduodenoscopy showed complete remission of the gastric MALT lymphoma, and colonoscopy showed a post-polypectomy scar (Fig. 2C). The patient is still alive without any recurrence of MALT lymphoma 36 months after diagnosis.
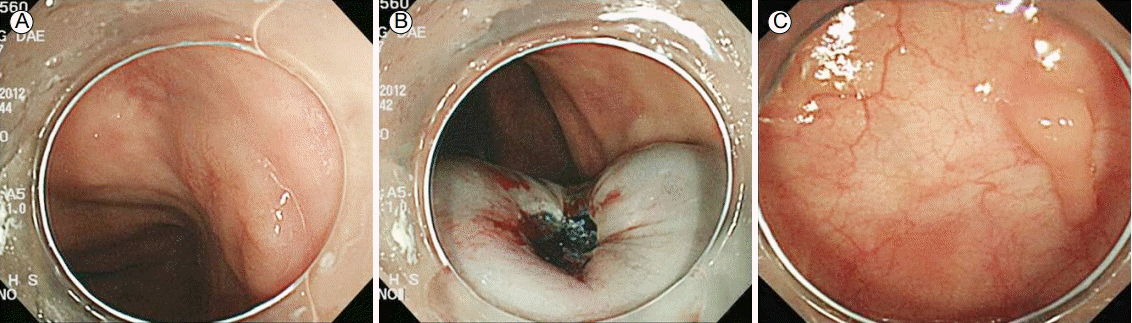
Colonoscopic findings. (A) A flat discolored 1.2-cm polyp is shown. (B) The polyp was removed using a snare, and the margins were clear. (C) Follow-up colonoscopy showing a polypectomy scar and an inflammatory polyp.

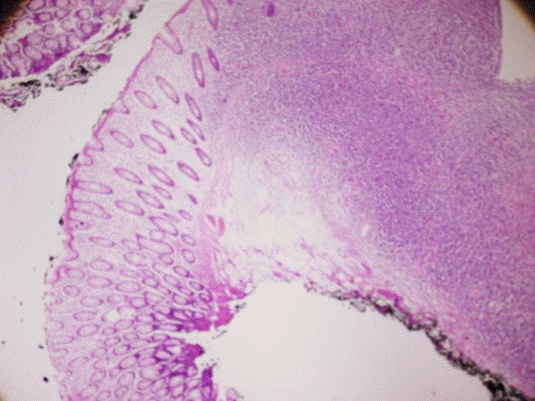
Pathologic findings. (A, B) Lymphoproliferative lesions in a colonoscopic biopsy are shown. (A, hematoxylin & eosin [H&E], ×100; B, H&E, ×400) (C, D) Immunohistochemical staining was positive for (C) Bcl-20 (×100) and (D) CD-20 (×100).
DISCUSSION
Primary extranodal MALT lymphoma occurs most commonly in the gastrointestinal tract; other sites include the lung, bladder, salivary gland, thyroid gland, breast, and skin [3]. Gastric MALT lymphoma is associated with H. pylori infection, and non-gastric MALT lymphoma (e.g., colonic MALT lymphoma) is associated with Borrelia burgdorferi, Chlamydia psittaci, Campylobacter jejuni and hepatitis C infection and autoimmune disease. However, the specific causative organism of colonic MALT lymphoma has not been identified. Gastric MALT lymphoma is asymptomatic or has nonspecific symptoms such as dyspepsia and epigastric pain. Primary colonic MALT lymphoma, which is very rare, is also asymptomatic or has nonspecific symptoms, such as abdominal bloating, diarrhea, and abdominal pain [4]. In endoscopy, colonic MALT lymphoma usually appears as a sessile protruding lesion or ulcerative lesion in sites such as the cecum (71.5%), rectum (16.9%), ascending colon (6.2%), and rarely the sigmoid colon [5].
Treatment protocols for gastric MALT lymphoma are well established but are less clear for colonic MALT lymphoma owing to its rarity. H. pylori eradication results in complete remission in 80% of patients with early-stage, low-grade gastric MALT lymphoma [6]. Even if H. pylori is not detected, anti-H pylori therapy is a viable treatment for gastric MALT lymphoma. However, the role of H. pylori in the development of colonic MALT lymphoma is unknown. Suggested treatments for colonic MALT lymphoma include chemotherapy, radiotherapy, anti-H. pylori therapy, endoscopic resection, and surgery; however, there is no consensus regarding the most effective therapy [7]. Raderer et al. [8] described a case of simultaneous gastric and colonic MALT lymphoma, in which only the latter was responsive to anti-H. pylori therapy. Matsumoto et al. [9] reported regression of rectal MALT lymphoma in a patient positive for H. pylori after its eradication. These reports suggest that anti-H. pylori therapy, which is not invasive, may be an effective first treatment for colorectal MALT lymphoma. However, if there is no response to anti-H. pylori therapy, individual treatments should be based on the disease stage, presence and types of comorbidities, patient’s age, and other clinical factors. In our case, colonic MALT lymphoma was incidentally treated via endoscopic resection; however, anti-H. pylori treatment can be the first option. Because the colonic lesion was flat and detected via colonoscopy only a short time after radiotherapy, it is possible that it was initially overlooked; this would suggest that the MALT lymphomas in the stomach and colon were synchronous. Unlike previous reports in which synchronous gastric and colon MALT lymphomas were treated together, the lymphomas in our case were inadvertently treated separately. However, both radiotherapy for the gastric MALT lymphoma and endoscopic resection for the colonic MALT lymphoma were successful; therefore these strategies can be used to treat future cases.
In conclusion, this report describes a rare case of a primary gastric and colonic MALT lymphoma in an otherwise healthy subject. Results from this case suggest that colonoscopic examination may be helpful for patients diagnosed with gastric MALT lymphoma. Although the optimal management strategy for primary colonic lymphomas has not been established, we treated the patient via endoscopic resection. To date (36 months after diagnosis), there is no evidence of disease recurrence.