말기신질환 환자에서 발생한 미만성 폐골화증 2예
Diffuse Pulmonary Ossification Developing in Patients with End-stage Renal Disease
Article information
Trans Abstract
Diffuse pulmonary ossification (DPO) is a rare condition characterized by chronic metaplastic ossification of the lung parenchyma. DPO is associated with various underlying pulmonary, cardiac, and systemic diseases. However, to our knowledge, DPO has rarely been described in patients with end-stage renal disease undergoing hemodialysis. We describe two cases of DPO diagnosed in long-term hemodialysis patients. Awareness of this rare disorder is required for a better differential diagnosis of cases presenting with bilateral diffuse micronodular lesions, including calcific opacities.
INTRODUCTION
Diffuse pulmonary ossification (DPO) is a rare condition characterized by widespread bone formation throughout the lung parenchyma [1]. DPO generally has an asymptomatic or indolent disease course, but a differential diagnosis is often necessary because radiological findings have shown that DPO mimics various pulmonary disorders with more significant clinical consequences [2,3]. Although the pathogenesis of DPO is not fully understood, it is known to be idiopathic or associated with a variety of underlying pulmonary, cardiac, and systemic disorders [1,2,4]. However, to our knowledge, DPO has not been reported in chronic hemodialysis patients with end-stage renal disease (ESRD) in the English literature, in contrast to metastatic pulmonary calcification that is a common complication in these patients [1]. Here, we report two cases of DPO developing in hemodialysis patients with ESRD diagnosed by surgical and transbronchial lung biopsy, respectively.
CASE REPORT
Case 1
A 33-year-old man presented with abnormal findings on chest radiography. He complained of breathlessness on exertion (grade I on the modified Medical Research Council dyspnea scale). However, he did not experience significant changes in respiratory symptoms or signs. He had been undergoing hemodialysis for 7 years because of ESRD secondary to chronic glomerulonephritis, which was diagnosed 9 years previously. He was a never-smoker, and his medical history included hypertension, gout, and hyperparathyroidism secondary to ESRD. The findings of physical examination were unremarkable, except for mild pallor of his conjunctivae.
The results of laboratory investigations, including white blood cell counts, liver function tests, and measurements of erythrocyte sedimentation rate, C-reactive protein, calcium, alkaline phosphatase, and carcinoembryonic antigen, were normal. The following abnormal laboratory findings were observed: hemoglobin 8.7 g/dL, blood urea nitrogen 36.5 mg/dL, creatinine 8.6 mg/dL, N-terminal pro-brain natriuretic peptide (NT-proBNP) 933 (normal range: 0-97.3) pg/mL, and parathyroid hormone 478.9 (normal range: 15-65) pg/mL. The results of arterial blood gas analysis in room air were unremarkable. Electrocardiography showed normal sinus rhythm with a heart rate of 66 beats per minute.
Chest radiography showed bilateral diffuse reticulonodular densities (Fig. 1A). Subsequent high-resolution computed tomography (HRCT) of the chest demonstrated numerous micronodules scattered throughout both lung fields, predominantly in the subpleural area of the lower lobes (Fig. 2A and 2B). In the mediastinal window setting, some of the nodules showed calcified density. There was no mediastinal lymphadenopathy. Lung function tests were within normal limits, except for decreased carbon monoxide diffusion capacity (45% of the predicted value). Transthoracic echocardiography showed a mildly dilated left atrium (42 mm) but normal systolic function of the left ventricle. Pulmonary amyloidosis, miliary tuberculosis, sarcoidosis, metastatic lung cancer, or ESRD-associated metastatic pulmonary calcification was first considered as the cause of the bilaterally scattered numerous pulmonary nodules on chest HRCT. To obtain further information, bronchoscopic examination with transbronchial lung biopsy (TBLB) was then performed. Results of microbial tests in bronchial aspirates were all negative. TBLB was not satisfactory because of substantial bleeding after one biopsy attempt. Thus, thoracoscopic lung biopsy was performed in the basal segment of the right lower lobe. Microscopically, deposits of osteoid material with fatty marrow element, measuring 1-3 mm in diameter, were found predominantly within the alveolar space (Fig. 3A). No other pathological findings suggestive of amyloidosis, granuloma, malignancy, or pulmonary fibrosis were detected.
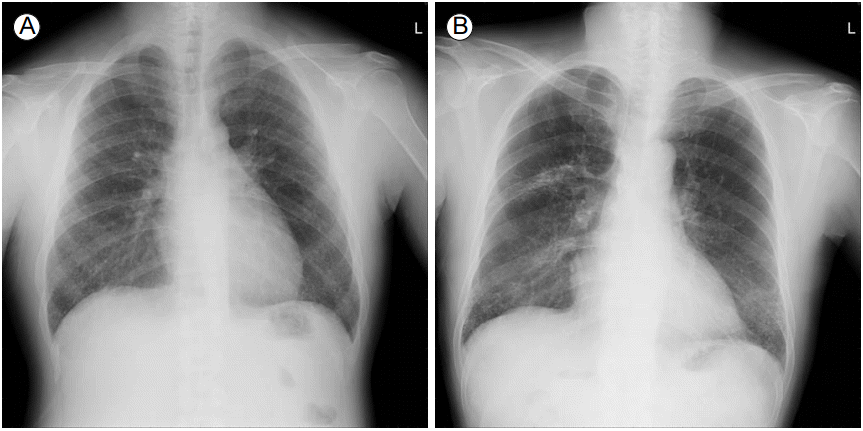
Chest radiography. Chest radiography showed bilateral diffuse reticulonodular densities (A: case 1; B: case 2), predominantly in the lower lung fields (B), and localized fibrocalcific densities in the right upper lung zone (B).

High-resolution computed tomography scans of the chest. Bilateral numerous nodular lesions were present in the lung window setting (A: case 1), and calcific densities (arrows) of some nodules were evident in the mediastinal window setting (B: case 1). Bilateral numerous branching lesions along interstitial septa were shown with basal predominance (C: case 2), and calcific opacities (arrows) were more evident in the mediastinal window setting (D: case 2).

Pathological findings. Histological findings showed ossified nodular lesions (arrowheads) with marrow elements (arrows) predominantly within the alveolar space in case 1 (A: hematoxylin and eosin [H&E] stain ×40; H&E stain ×100 for inset image) and bone tissue fragment (arrowheads) in the peripheral parenchyma in case 2 (B: H&E stain ×40; H&E stain ×100 for inset image).
Case 2
A 65-year-old male hemodialysis patient with ESRD due to diabetic nephropathy was referred to the pulmonology department for evaluation of a radiological abnormality, which was found during a recent hospitalization for a gait disturbance that was associated with normal-pressure hydrocephalus 2 weeks previously. He complained of generalized weakness and was an ex-smoker with a 40-pack-year history. He had regularly undergone hemodialysis three times a week for 8 years, and his medical history included diabetes mellitus, hypertension, and angina pectoris.
At the time of admission for evaluation, his vital signs were stable and no heart murmurs or abnormal lung sounds were found on physical examination. Abnormal laboratory findings were as follows: blood urea nitrogen 38.7 mg/dL, creatinine 7.44 mg/dL, calcium 11.1 (normal range: 8.6-10.2) mg/dL, NT-proBNP 10,765 pg/mL, and parathyroid hormone 72.41 pg/mL. The results of other laboratory investigations, including white blood cell counts, liver function tests, and measurements of C-reactive protein, sodium, and calcium, were normal. Electrocardiography showed normal sinus rhythm with a heart rate of 71 beats per minute.
Chest radiography showed bilateral diffuse reticulonodular opacities, predominantly in the lower lung fields, and localized fibrocalcific densities in the right upper lung zone (Fig. 1B). HRCT demonstrated innumerable nodular and branching calcific opacities along the interstitial septa with basal predominance (Fig. 2C and 2D). A whole-body bone scan with technetium-99m hydroxymethylene diphosphonate (Tc-99m HMDP) revealed diffusely increased uptake of tracer in both lungs (Fig. 4). The results of pulmonary function tests were also within normal limits, except for decreased diffusion capacity (66% of the predicted value). Histopathological examination of specimens obtained from the left lower lobe through TBLB showed a fragment of bone tissue (Fig. 3B). We decided not to perform thoracoscopic or open lung biopsy to further confirm the histological findings because of multiple risk factors, including the patient’s neurological symptoms. Based on the available radiological, bone scintigraphic, and histological findings, we assumed that the patient had DPO.
DISCUSSION
Here, we presented two rare cases of DPO developing in hemodialysis patients with ESRD who had bilateral diffuse reticulonodular opacities on plain radiographs and numerous micronodules, including calcified densities, predominantly in the lower lobes on HRCT. This report provides additional information regarding DPO as a potential differential diagnosis based on the above-mentioned radiological findings in hemodialysis patients with ESRD.
The mechanism responsible for DPO is thought to involve the transformation of pulmonary fibroblasts into osteoblasts as a result of chronic lung insult [1,2,4]. This disease is generally classified as either nodular or dendriform DPO, based on its histological characteristics [1]. It has been suggested that the etiologies of such metaplasia may differ between these two types of DPO. As nodular DPO is classically found in patients with mitral stenosis and occurs pathologically within the intraalveolar space, it is considered to be a consequence of alveolar exudates due to secondary congestion [4]. In contrast, dendriform DPO involving the alveolar septa is usually associated with chronic inflammation, such as pulmonary fibrosis or other chronic lung disease [4,5]. However, no clinical association is found in some cases, particularly in the dendriform type [3]. Moreover, some patients may show both histological features simultaneously [2,6]. In our two cases, the main histological features appeared to be different. In our first case, the radiological and pathological findings indicated that the patient had, predominantly, nodular DPO, which was characterized by osseous tissue developing within the alveolar space (Fig. 3A). However, most of this osseous tissue contained marrow elements, which are characteristic of dendriform DPO [2]. This patient had been undergoing regular hemodialysis due to underlying ESRD, but did not have any underlying pulmonary disease. We could not determine whether these overlapping histological features (both nodular and dendriform characteristics) were attributable to ESRD-hemodialysis, because there is a lack of evidence regarding how ESRD-hemodialysis affects the development of DPO. Subsequent reports of DPO cases in hemodialysis patients with ESRD are likely to provide more information that will contibute to resolving this issue. In our second case, we assumed that the patient had, predominantly, dendriform DPO based on the radiological findings, which showed branching opacities characteristic of this type [6]. Our findings suggest that both the nodular and dendriform types of DPO can occur in hemodialysis patients with ESRD.
Radiological findings, especially HRCT, are the most important factors for suspicion of DPO. As in the two cases reported here, small nodular or branching opacities are found on HRCT (range: 1-3 mm), with a bilateral diffuse distribution, and are predominantly located in the lower lobes [7,8]. Although a few nodules had high-density opacities suggesting calcification or bony lesions, in the mediastinal window setting of HRCT scans, such high-density opacities may not be apparent in most nodules, probably because of their small size. In contrast to DPO, metastatic pulmonary calcification in hemodialysis patients is characterized by numerous nodules measuring 3-10 mm in size predominantly located in the lung apices [9].
Although DPO generally has an indolent course, with minimal clinical significance [6], DPO may mimic other serious conditions requiring timely appropriate treatment [1]. If not diagnosed based on histological findings, DPO can be misdiagnosed as other diseases [2,3]. Therefore, additional diagnostic steps, such as pathological examinations, are recommended to ensure an accurate diagnosis in patients presenting with the above-mentioned radiological findings.
Although most previous DPO cases were confirmed by autopsy or surgical lung biopsy, Peros-Golubicić and Tekavec-Trkanjec [6] reported one case of DPO diagnosed by TBLB. They reported that rigid specimens did not pass easily through the working channel of the bronchoscope. However, we did not experience any particular resistance during TBLB when using a bronchoscope with a wide working channel (2.8 mm). Although large lung specimens are essential for precise histological confirmation of DPO, these findings suggest that TBLB may be an alternative method for a presumptive diagnosis and for exclusion of other potential diseases, when accompanying consistent evidence from chest CT and Tc-99m HMDP bone scan, in patients with risk factors [1,10].
Here, we presented two cases of DPO, one of which had a distinct histological subtype, which developed in patients with ESRD-hemodialysis. It is important for clinicians to be aware of this DPO entity in a differential diagnosis based on radiological findings showing bilateral diffuse micronodules, including calcific densities.
