약제 내성 만성 B형 간염 환자에서 S202, M250 엔테카비어 내성이 테노포비어 근간 구제 요법의 바이러스 반응에 미치는 영향
Entecavir Resistance at rtS202, rtM250 May Cause Poor Viral Response to Tenofovir-based Rescue Therapy in Chronic Hepatitis B
Article information
Abstract
목적:
만성 B형 간염 치료에서 장기적인 항바이러스제 치료는 약제 내성 발현을 유도할 수 있다. 이번 연구는 다약제 내성 및 기존 약제 내성에 대한 구제 요법에 부분 바이러스 반응을 보이는 만성 B형 간염 환자를 대상으로 테노포비어 근간 구제요법의 효과를 분석하고자 하였다.
방법:
총 54명의 다약제 내성 환자를 대상으로 후향적 분석을 시행하였다.
결과:
대상 환자는 26가지의 다양한 변이를 가지고 있었고, 76%가 1년 내에 완전 바이러스 반응을 보였으며, 다변량 분석에서 치료 전 혈청 HBV-DNA 값이 20,000 IU/mL, HBeAg 음성은 완전 바이러스 반응을 보이는 예측인자였다. 흥미롭게도 S202 위치의 변이 여부가 바이러스 반응과 관련이 있었다.
결론:
다약제 내성 및 기존 약제 내성에 대해 부분 바이러스 반응을 보이는 만성 B형 간염 환자에서 치료 전 낮은 HBV-DNA 수치, HBeAg 음성 여부, S202 변이가 없을 때 완전 바이러스 반응을 유도하는 경항을 보였다.
Trans Abstract
Background/Aims:
Long-term use of nucleos(t)ide analogues (NA) may lead to genotypic and/or phenotypic resistance of the hepatitis B virus (HBV). We investigated the efficacy of tenofovir-based rescue therapy in chronic hepatitis B (CHB) patients with newly developed genotypic resistance to prior NAs or partial virologic response to sequential rescue therapies.
Methods:
Fifty-four CHB patients were included retrospectively. The patients were treated with tenofovir alone or combined with lamivudine or entecavir.
Results:
There were 26 forms of genotypic resistance at enrollment. The median amount of serum HBV-DNA was 18,438 IU/mL and 83% of samples were positive for hepatitis B e antigen (HBeAg). Serum HBV-DNA was undetectable in 50%, 61%, and 76% of the patients at 3, 6, and 12 months, respectively. In multivariate analysis, HBV-DNA < 20,000 IU/mL and negative HBeAg at baseline were independent predictors of negativity for serum HBV-DNA. Interestingly, the rtS202 mutation tended to be associated with an unfavorable response. Other clinical variables and viral resistance genotypes showed non-significant viral response.
Conclusions:
Lower serum HBV-DNA, negative HBeAg and lack of rtS202G mutations at baseline may predict a favorable response to tenofovir-based rescue therapies in CHB patients with newly developed genotypic resistance to prior NAs or a partial virologic response to sequential rescue therapies.
INTRODUCTION
Nucleos(t)ide analog (NA) antivirals effectively control chronic hepatitis B (CHB) to prevent or retard the progression of chronic liver disease, portal hypertensive complications and hepatocellular carcinoma (HCC) [1,2]. The long-term maintenance of hepatitis B virus (HBV) DNA suppression by NAs is an important factor that can slow the progression of liver diseases [3-6]. The long-term use of NAs, particularly less potent and lower genetic barrier drugs such as lamivudine (LMV) and adefovir (ADV), has increased the likelihood of drug resistance in CHB patients in proportion to the duration of treatment, resulting in treatment failure [1,2]. CHB is highly prevalent in Korea, and the newer and more potent NAs, entecavir (ETV) and tenofovir (TDF), were unavailable until 2007 and 2012, respectively. Therefore, the occurrence of genotypic resistance due to use of less-potent NAs has been a primary issue in the management of CHB patients. Furthermore, sequential rescue therapies for CHB patients with genotypic resistance can lead to multidrug resistant HBV strains due to sequential selection of mutations that cannot be effectively suppressed by antivirals; this also contributes to the progression of chronic liver diseases [7,8].
TDF has the most potent antiviral activity and safety profile among NAs in CHB-naïve patients [9]. However, few studies describe the efficacy of TDF-based rescue therapy against partial virologic response (PVR) with genotypic resistance developing after long-term LMV, ADV, ETV or combination therapies. It is also unclear whether any form of genotypic resistance is associated with the efficacy of TDF. Thus, we investigated the antiviral efficacy of TDF-based rescue therapy in CHB patients who had newly developed resistance to prior NAs or PVR caused by genotypic resistance, and attempted to identify the viral factors associated with a favorable response.
MATERIALS AND METHODS
Patients and study design
A total of 54 CHB patients with newly developed genotypic resistance to NAs, or PVR to previous antiviral therapies, were enrolled retrospectively at Hallym University Medical Center, Korea, from December 2012 to September 2013. The criteria for enrollment were 1) adherence to previous NA treatment, 2) measurable baseline serum HBV DNA at the time of TDF-based rescue therapy, and 3) genotypic resistance with newly developed genotypic resistance or PVR to the previous therapies. None of the patients were co-infected with hepatitis C virus, human immunodeficiency virus, or hepatitis D virus and they did not have other concomitant liver diseases such as alcoholic liver cirrhosis, autoimmune hepatitis, or HCC. The study protocol was approved by the institutional review boards of each institution and followed the ethical guidelines of the 1975 Declaration of Helsinki.
Definition of partial virologic response
PVR was defined as a decrease in serum HBV DNA > 1 log10 IU/mL or copies/mL with detectable serum HBV DNA after 6 months of prior antiviral therapy maintenance [1]. Viral breakthrough was defined as an increase > 1 log10 IU/mL or copies/mL in HBV DNA from baseline [2].
Clinical and laboratory assessments
Serum alanine aminotransferase (ALT), albumin, bilirubin, creatinine, creatinine clearance, hepatitis B surface antigen (HBsAg), antibody to HBsAg (anti-HBs), hepatitis B e antigen (HBeAg), antibody to HBeAg (anti-HBe), and serum HBV DNA (lower limit of detection of 20 IU/mL) were measured every 3 months by the investigators. Laboratory data were also obtained from the medical records. Genotypic resistance to antivirals such as LMV, ADV, and ETV was determined using restriction fragment mass polymorphism at initiation of TDF-based rescue therapy.
Study objectives and endpoints
The primary study objective was to investigate the efficacy, measured as viral response (VR), of TDF-based rescue therapy in NA-experienced patients with genotypic resistance. Therefore, the primary end point was defined as serum HBV DNA negativity (< 20 IU/mL) after 3, 6, and 12 months of TDF-based rescue therapy. Secondary endpoints were ALT normalization, HBeAg loss or seroconversion and emergence of genotypic resistance to TDF.
Statistical analyses
Serum HBV DNA levels were logarithmically transformed for analyses. Categorical variables were analyzed using the χ2-test, and continuous variables were analyzed using the Mann-Whitney U-test, as appropriate. The Kaplan-Meier methodology was used to evaluate the cumulative rate of HBV DNA negativity, and subgroup comparisons were performed using the log-rank test. The Cox proportional hazards model was used to evaluate the factors independently associated with VR. A P-value < 0.05 was considered to indicate statistical significance. Statistical analyses were performed using SPSS for Windows software (ver. 21.0; SPSS Inc., Chicago, IL, USA).
RESULTS
Patient baseline characteristics
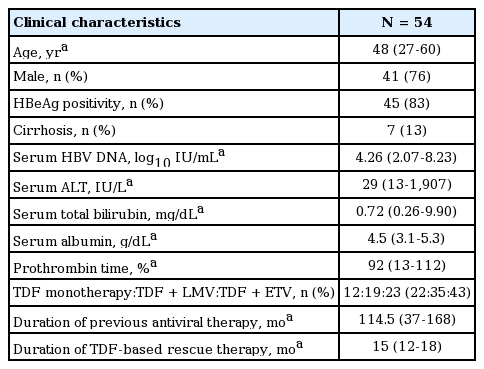
The baseline characteristics of all patients (n = 54) are summarized in Table 1. Forty-one (76%) patients were male, with a median age of 48 years (range: 27-60 years). Seven patients (13%) were cirrhotic and forty-five (83%) were HBeAg positive. Patients were followed for a median of 15 months (range: 12-18 months) using TDF-based rescue therapies. Previous antiviral therapies were administered for a median of 114.5 months (range: 37-168 months) and the median HBV DNA level prior to TDF-based rescue therapy was 4.26 log10 IU/mL (range: 2.07-8.23 log10 IU/mL). TDF-based rescue regimens were subclassified as TDF monotherapy, TDF with LMV combination therapy, and TDF with ETV combination therapy (11, 20, and 23 patients, respectively).
Previous antiviral treatments and resistance profiles
Sequential treatment regimens for each patient are shown in Figure 1. Fifty-two patients (96%) had been treated with LMV; only two patients had been treated with ETV before TDF-based rescue therapy. Subsequently, TDF monotherapy was applied as a first-line therapy in three (27%) patients, as a second-line therapy in three (27%) patients, and as a third-line rescue therapy in five (54%) patients. When combined, TDF with LMV were the first-line therapy in six (30%) patients, a second line therapy in eight (40%) patients, a third-line therapy in three (15%) patients and a fourth-line rescue therapy in three (15%) patients. Finally, TDF with ETV was used as a second-line therapy in five (22%) patients, a third-line therapy in ten (43%) patients, a fourth-line therapy in six (26%) patients and a fifth-line rescue therapy in two (9%) patients. The patterns of baseline genotypic resistances at the start of TDF-based rescue therapy are detailed in Table 2. All patients were infected with an HBV strain with rtM204.

Antiviral drug regimens before and after the emergence of resistance. LMV, lamivudine; ETV, entecavir; ADV, adefovir; TDF, tenofovir.
Virologic response
Figure 2. shows the changes in mean HBV DNA levels during the first 12 months. Following initiation of TDF-based rescue therapy, serum HBV DNA levels declined continuously; the overall changes in median values at 3, 6 and 12 months were -2.37, -2.72 and -2.88 log10 IU/mL, respectively. During the first year of TDF rescue therapy, HBeAg loss occurred in 6.7% (3 of 45) of HBeAg-positive patients, and one patient had HBeAg seroconversion.

Changes in mean hepatitis B virus DNA levels from baseline to month 12. The overall mean changes were -2.37, -2.72 and -2.88 log10 IU/mL at 3, 6 and 12 months, respectively. HBV DNA, hepatitis B virus DNA.
Cumulative probabilities of achieving VR were 50%, 61% and 76% at 3, 6 and 12 months, respectively (Fig. 3A). They were higher in the patients without ETV-resistant HBV than in the patients with ETV-resistant HBV (59%, 73% and 87% vs. 29, 35% and 53%, respectively, at 3, 6 and 12 months, p = 0.002). Interestingly, cumulative probabilities of achieving VR were significantly higher in the rtS202 non-mutant group than in the rtS202 mutant group (55%, 68% and 84% vs. 14, 14% and 29%, respectively, at 3, 6 and 12 months, p = 0.005, Fig. 3B). Also, two patients with the rtM250L/V mutation tended to show poor VR during follow-up, although the difference was not statistically significant (p = 0.057). Taken together, the patients with rtS202G or rtM250L/V, entecavir resistant genotypes, were significantly associated with failure to achieve VR (58%, 71% and 88% vs. 11%, 11% and 22%, respectively, at 3, 6 and 12 months, p < 0.001). Other genotypic resistances to ETV, e.g., rtI169T, rtV173L and rtT184A/I/L, not rtS202G and rtM250L/V, showed similar responses compared to patients with other genotypic resistances (p = 0.638). The HBV-DNA level was significantly higher in the group with < 20,000 IU/mL baseline serum HBV-DNA than in that with > 20,000 IU/mL (75%, 82% and 86% vs. 23%, 38% and 65%, respectively, at 3, 6 and 12 months, p = 0.007, Fig. 3C). HBeAg was more common in the HBeAg-negative group than in the HBeAg-positive group (78, 100 and 100% vs. 4, 53% and 72%, respectively, at 3, 6 and 12 months, p = 0.004, Fig. 3D).
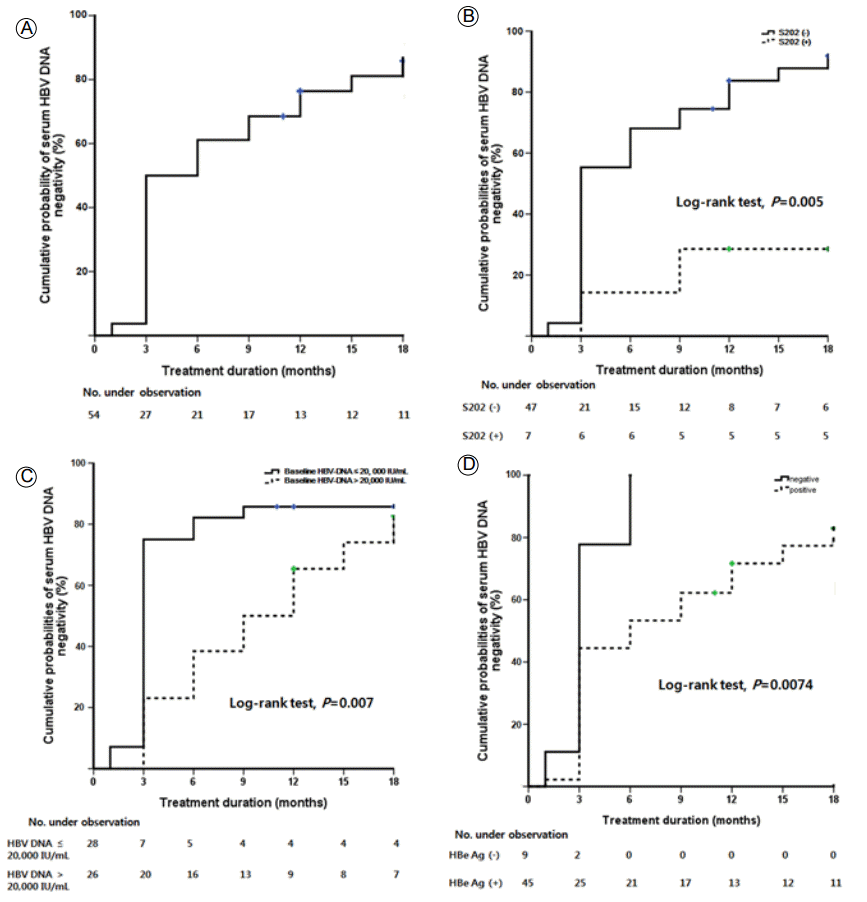
(A) Cumulative virologic response rates were 50%, 61% and 76% at 3, 6, and 12 months, respectively. (B)Cumulative virologic response rates in the S202 non-mutant group were significantly higher than those in the S202 mutant group (p = 0.005 by the log-rank test). (C) Cumulative virologic response rates in the group with a baseline serum hepatitis B virus-DNA level of ≤ 20,000 IU/mL were significantly higher than those in the > 20,000 IU/mL group (p = 0.007 by log-rank test) (D) Cumulative virologic response rates in the hepatitis B e antigen-negative group were also significantly higher than those in the hepatitis B e antigen-positive group (p = 0.0074 by the log-rank test). HBV DNA, hepatitis B virus DNA; No., number; HBeAg, hepatitis B e antigen.
Favorable predictive factors for viral response (VR) with TDF-based rescue therapy
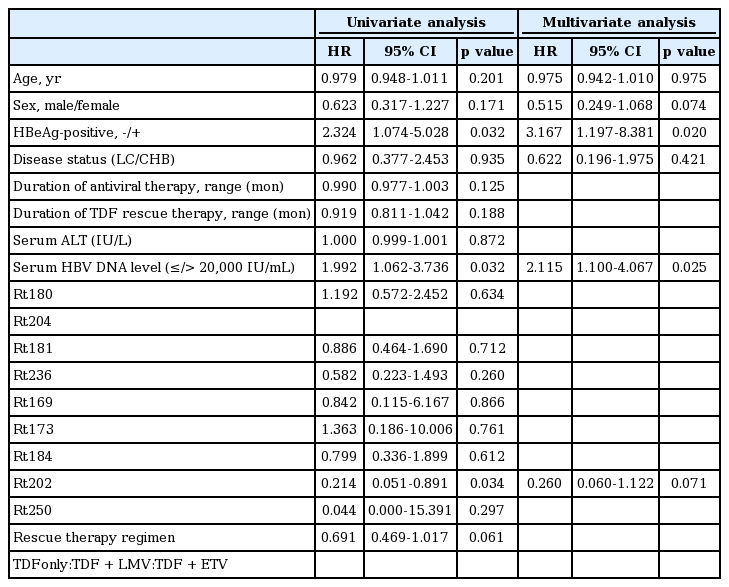
A multivariate Cox regression model was used to identify the independent risk factors significantly associated with VR during the follow-up period. In univariate analysis, the rtS202 mutant, baseline serum HBV DNA level and negative HBeAg were candidate variables for multivariate analysis (p < 0.05). According to genotype, the group without rtS202 or rtM250 mutants was highly associated with achieving VR (harzard ratio [HR] 0.149; 95% CI: 0.036-0.629; p = 0.009). This was also true on multivariate analysis (HR 0.201; 95% CI: 0.046-0.878; p = 0.033). Other clinical factors such as age, gender, presence of cirrhosis, duration of antiviral therapy, duration or regimen of TDF rescue therapy, laboratory variables, and type of genotypic resistance other than rtS202, were not significantly associated with VR. In multivariate analysis, a lower baseline serum HBV DNA (≤ 20,000 IU/mL) and negative HBeAg were independent predictors of VR (p < 0.05). Despite the small number of patients (n = 7), the rtS202 mutation tended to be strongly associated with unfavorable VR (p = 0.07) (Table 3).
Safety and side effects
TDF-based rescue therapy was well tolerated and no clinically relevant side effects were noted. The serum creatinine level remained unchanged at 0.85 mg/dL (range: 0.5-1.1 mg/dL) at baseline and 0.85 mg/dL (range: 0.4-1.1 mg/dL) at 12 months. No other laboratory abnormalities were reported during the study.
DISCUSSION
The present study revealed that TDF-based rescue therapy effectively suppressed HBV, even in CHB patients with PVR caused by genotypic resistance to previously used antiviral drugs. In practice, the cumulative rate of undetectable serum HBV DNA at 12 months reached ~76%, indicating that TDF has similar antiviral efficacy in CHB-naïve patients. Recent studies also demonstrated that TDF-based rescue therapy may effectively suppress viral replication, even in patients with multidrug-resistant HBV or PVR to previous antiviral therapies. Even TDF monotherapy could provide VR comparable to that seen with TDF with ETV combination therapy in those patients [10-16].
NAs with lesser potency and a lower genetic barrier may give rise to genotypic resistance, especially with long-term use. Moreover, sequential NAs monotherapy or combination therapies promote multidrug resistance, and consequently demonstrate insufficient suppression of HBV replication. In this study, LMV was administered as a first-line therapy in 52 patients (96%) in whom viral breakthrough and LMV-resistance developed, as expected. In 34 patients (65.4%) treated with ADV alone or ADV combined with LMV as the second-line rescue therapy, HBV suppression was ineffective. However, the replication of LMV-resistant HBVs was suppressed effectively when the rescue therapy was designed on the basis of TDF. Favorable VR was observed in ETV-resistant HBVs even when they were not mutants of rtS202 or rtM250.
A previous in vitro study showed that rtA181T/V or rtN236T may decrease susceptibility to TDF fourfold, but had little effect on ETV [17]. However, in this study, the patients with rtA181T/V or rtN236T showed a similar VR to those with other genotypic resistance treated with TDF rescue regimens (p = 0.292). In addition, five of nine patients with rtA181T/V and rtN236T in our study achieved VR, although a previous study reported that CHB patients with these mutations were refractory to TDF monotherapy or TDF with LMV combination therapy [10]. In particular, two patients with these mutations achieved VR within 9 months of TDF monotherapy, and another four of seven patients achieved VR within 12 months of using TDF and ETV combination therapy. Lim et al. reported that the patients who had a single ADV-resistance mutation or double ADV-resistance mutations (rtA181T/V or/and rtN236T) showed similar VR to each treatment regimen, TDF monotherapy, or TDF with ETV combination therapy [14]. Therefore, any TDF-based rescue therapy may be effective in patients with HBV at rtA181T/V and/or rtN236T strains, although further evaluation with a large number of cases is warranted.
As expected, the baseline serum HBV DNA level was an independent predictor of VR. Several studies have shown that a lower baseline serum HBV DNA prior to therapy is strongly associated with antiviral efficacy measured by viral suppression, HBeAg loss or seroconversion, serum ALT normalization and histological findings [18-22]. Poor VR to antiviral agents is observed primarily in CHB patients with high baseline serum HBV-DNA levels and HBeAg positivity [23,24]. In the present study, a serum HBV DNA level < 20,000 IU/mL at pre-treatment could be used as a cut-off for predicting achievement of VR with TDF-based rescue therapy. Negative HBeAg was also an independent factor predicting favorable VR in CHB patients with genotypic resistance. In fact, all HBeAg-negative CHB patients showed undetectable serum HBV-DNA within 6 months. These findings suggest that patients negative for HBeAg, or who had lower baseline serum HBV-DNA levels, may achieve VR more easily than those positive for HBeAg or with higher baseline serum HBV-DNA levels.
One of the most interesting findings of this study is that the cumulative probabilities of achieving VR were significantly lower in patients with the rtS202 mutation than in those without the rtS202 mutation (29% vs. 84% at 12 months, p = 0.005). Also, two patients with the rtM250 mutation and one patient with mutations of both rtT184 and rtS202G did not achieve VR within 12 months. However, other ETV-resistance strains of HBV without rtS202 or rtM250 did not show a difference in VR for TDF-based rescue therapy. According to a previous report, the rtT184, rtS202, and rtM250 mutations may affect VR for antiviral therapy by impacting the sequence of the overlapping HBV surface antigen gene [25], although the association of these mutations with VR in TDF-based rescue therapy requires clarification. Interestingly, the patients with mutations of both rtT184 and rtS202 or rtM250 alone did not achieve VR during follow-up. The changes at rtT184 or rtS202 act by repositioning the YMDD loop and affecting the size of the ETV-triphosphate binding pocket; these substitutions then change the stabilizing H-bonding network between rtT184 and rtS202. A previous study found that the isolate containing both rtT184 and rtS202 substitutions displays the highest level of ETV resistance [25,26]. Our study also showed that one patient with the HBV mutation at rtT184 and rtS202 did not achieve VR. Substitution of rtM250 is unique in that resistance appears to reside primarily in minus-strand DNA synthesis, suggesting that contacts with the primer-template may be involved in resistance. The experiments showed that mutations of rtM250 and both rtT184 and rtS202 significantly more decreased nearly equivalent manner for ETV susceptibility compared with a single mutation at rtT184 or S202 [25]. Genotypic analysis of the ETV-resistant HBV revealed that 89% of ETV-resistant HBV had mutations at rtT184, rtS202, or both, with 17% having a combination of the two. Only 11% had mutations at rtM250, alone (5.5%) or in combination with those at rtT184 or rtS202 (5.5%). These findings suggest that a mutation at rtM250 is rarer than that at rtT184 and rtS202 [27]. Interestingly, a mutation at rtM250 results in phenotypic resistance to ETV [28]. Although the mutations at rtT184 or rtS202 may affect the YMDD loop position, our results suggest that the patients with a mutation at rtT184 showed favorable VR compared with other patients but the patients with a mutation at rtS202 showed unfavorable VR. This suggests that the existence of mutations at both rtT184 and rtS202, rtS202 alone or rtM250 alone should be considered when VR is predicted in a clinical setting, although the number of patients with these mutations was too small to draw a definite conclusion. Therefore, further long-term follow-up may determine a method of suppressing HBV with rtT184, rtS202, and rtM250 mutations in a large population.
Other prospective clinical trials of TDF and TDF with ETV rescue therapy in ADV-resistant or ETV-resistant patients have shown that 48 weeks of TDF monotherapy provided a comparable rate of VR compared with TDF and ETV combination therapy [14,15]. Our study showed similar results to the above trials. TDF monotherapy in particular may be a reasonable treatment option for multidrug resistant CHB patients without mutations at rtS202, rtM250, or both rtS202 and rtT184.
There were several limitations to our study. First, the sample size was too small to conclude that TDF-based rescue therapy is the only option for CHB with PVR with genotypic resistance to prior therapies. However, it should be considered that the inclusion of a large number of such cases is impractical. It was also difficult to enroll more patients due to the shortage of multidrug-resistant CHB patients using TDF as a first-line therapy. Second, baseline genotypic resistance was heterogeneous and TDF-based rescue regimens varied according to the clinicians’ preference. Third, the follow-up period was relatively short; therefore, a study of prolonged duration would be required to investigate long-term efficacy. Finally, the study used a retrospective observational design. Thus, a large-scale prospective study should be conducted in future, although patients who develop new resistance to NAs and have partial response to sequential rescue therapies are relatively rare.
In conclusion, TDF-based rescue therapy appeared to be effective in patients who had newly developed genotypic resistance to prior NAs or PVR to sequential rescue therapies. A serum HBV-DNA level of < 20,000 IU/mL, HBeAg negativity, and S202G non-mutations at baseline may be predictive of VR with TDF-based rescue therapy. TDF monotherapy in particular may be a reasonable treatment option for multidrug resistant CHB patients without mutations at rtS202, rtM250, or at both rtS202 and rtT184.