중간정도 협착을 보이는 관상 동맥에서 발생한 급성심근경색
Acute Myocardial Infarction Occurring at a Preexisting Intermediate Coronary Artery Stenosis
Article information
Trans Abstract
Acute myocardial infarction often evolves from a mild coronary lesion. Therefore, the evaluation and management of intermediate coronary stenosis are important to prevent cardiac events. However, the decision on how to treat these lesions is challenging. Here, we report acute myocardial infarction occurring at a preexisting intermediate coronary stenosis based on invasive coronary angiography performed 10 days before the event.
INTRODUCTION
Coronary artery disease (CAD) is a leading cause of mortality worldwide. Invasive coronary angiography (ICA) is the gold standard in the diagnosis of CAD. However, when ICA reveals an intermediate coronary stenosis, the decision on how to treat this lesion is challenging [1]. Importantly, many cases of acute myocardial infarction (AMI) are believed to occur in mild coronary stenosis. However, angiographic detection of vulnerable plaque in a lesion with mild luminal narrowing is difficult, and little is known about the progression time from mild or intermediate stenosis to an occlusive lesion causing a coronary event. Here, we describe a patient who suffered AMI because of a lesion with intermediate coronary stenosis, as confirmed by ICA 10 days before the AMI.
CASE REPORT
A previously healthy 59-year-old man visited our outpatient clinic with a 2-week history of anterior chest pain. The chest pain occurred regardless of his activity, was triggered by position change, persisted for 30 seconds, and was then relieved spontaneously. There were no accompanying symptoms like dyspnea, fever, cough, or chills. He had no history of hypertension, diabetes mellitus, or dyslipidemia. He was a 40-pack-year ex-smoker. His vital signs were stable (blood pressure, 100/60 mmHg; heart rate, 67 beats/minute; body temperature, 36.4°C), and laboratory test results were within normal ranges, including a fasting glucose of 96 mg/dL and a low-density lipoprotein (LDL) cholesterol of 98 mg/dL. Chest radiographs and electrocardiograms were normal. Transthoracic echocardiography showed good left ventricular systolic function without regional wall motion abnormalities. Coronary computed tomography angiography (CCTA) revealed mild discrete stenosis (30%) with calcified plaques in the proximal left anterior descending coronary artery (LAD) and mild discrete stenosis (30%) with non-calcified plaques in the proximal left circumflex coronary artery (LCX). The right coronary artery (RCA) was normal. There was no evidence of aortic dissection or pulmonary embolism on CCTA. Gastroduodenoscopy showed no noteworthy finding, except superficial gastritis. Based on these results, the chest pain was considered nonspecific and he was followed without medication.
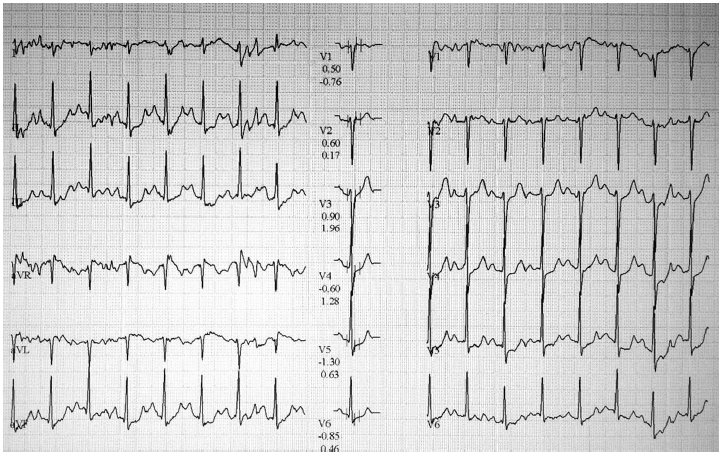
During follow-up, his chest pain worsened slightly. During an exercise treadmill test, there was no chest pain, but there was 2-mm horizontal ST-segment depression in leads II, III, aVF, and V4 to V6 at stage 2 of the Bruce protocol (Fig. 1). ICA was performed based on the exercise treadmill test result and persistent chest pain. There was an intermediate focal stenosis (stenosis diameter of 49% by quantitative coronary angiography) in the proximal LCX. The LAD looked normal, and the RCA was hypoplastic without stenosis (Fig. 2). Since the proximal LCX lesion was considered insignificant, no further testing or intervention was performed. After ICA, aspirin 100 mg was prescribed, and he was discharged from the hospital.
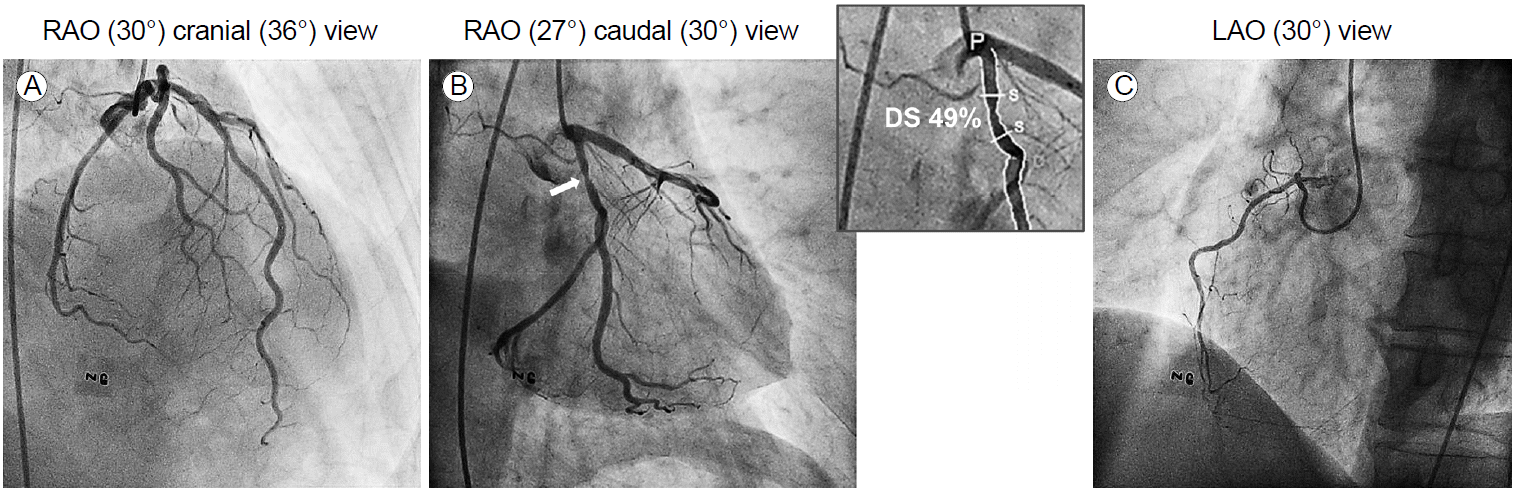
Invasive coronary angiography showing (A) no significant luminal stenosis in the left anterior descending artery, (B) intermediate stenosis in the proximal left circumflex artery (white arrow), and (C) a hypoplastic right coronary artery without stenosis. RAO, right anterior oblique; LAO, left anterior oblique; NG, nitroglycerin; DS, diameter stenosis; P, proximal; S, stenosis; D, distal.
Ten days later, he visited our emergency department with severe anterior chest pain at rest that persisted for more than 30 minutes. His blood pressure was 114/69 mmHg and pulse rate was 62 per minute. An electrocardiogram showed markedly elevated ST-segments in leads II, III, and aVF, with reciprocal ST-segment depression in leads V1 to V4 (Fig. 3). Emergency ICA revealed total thrombotic occlusion in the proximal LCX (Fig. 4A). First, a thrombectomy was performed using aspiration catheters, and much thrombi was aspirated (Fig. 4B). Then, a drug-eluting stent (3.0 × 18 mm, Resolute Integrity; Medtronic, Minneapolis, MN, USA) was implanted (Fig. 4C). He was stable after the procedure and discharged from the hospital 5 days later. Subsequently, his chest pain has not recurred.
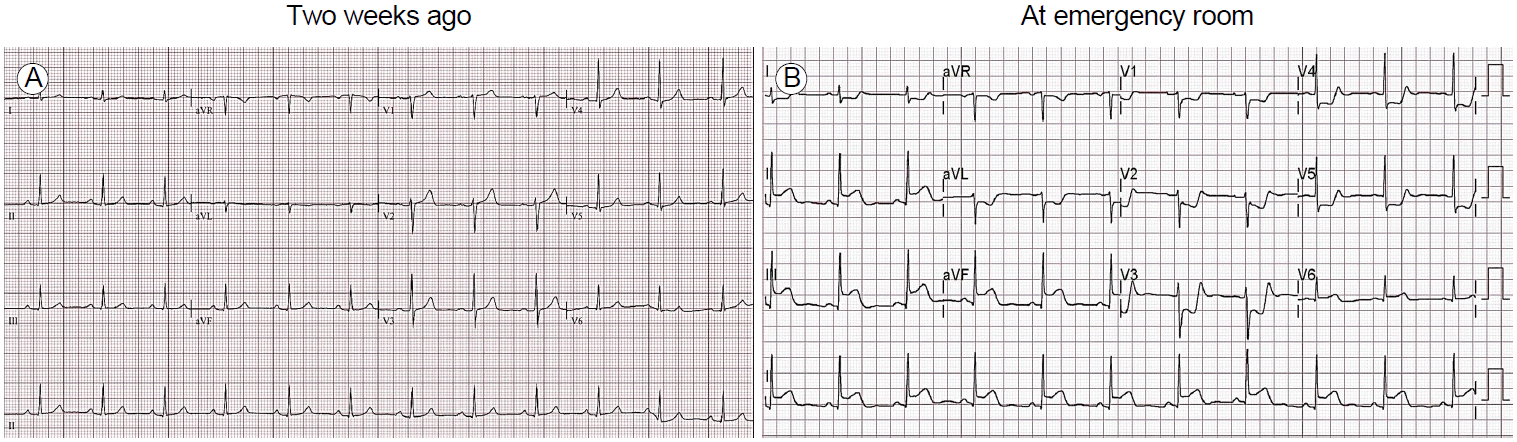
Electrocardiographic changes from normal findings before presentation (A) to acute myocardial infarction in the emergency department (B).
DISCUSSION
ICA is the gold standard for evaluating coronary status. It provides information about the presence, location, and severity of CAD with excellent spatial and temporal resolution, and offers immediate therapeutic intervention. However, it is difficult evaluate to decide on revascularization in an intermediate coronary lesion, defined as 40% to 70% stenosis of the coronary luminal diameter [1]. In particular, the anatomical assessment of luminal narrowing by ICA provides no insight into the vessel wall characteristics, including plaque deposition and morphology. Since AMI sometimes originates from a mild or intermediate coronary lesion, it is important to predict which coronary angiographic lesion is likely to cause AMI [2]. In order to overcome these limitations of ICA, the fractional flow reserve (FFR) or intravascular ultrasound (IVUS) during intervention has been used to predict whether a lesion will cause symptoms and to decide on how to manage the lesion [3]. In our case, the atypical nature of the chest pain and insignificant CCTA results made us overlook an intermediate stenosis in the proximal LCX on ICA, although the excise treadmill test was positive. If FFR or IVUS was performed at the initial ICA, hemodynamic significance or unstable plaque characteristics may have been revealed. Therefore, it is possible that AMI could be prevented by more appropriate management.
The failure to administer optimal medical therapy to patients with intermediate coronary stenosis is an important issue. With advances in medical therapy, current medical treatment is very effective and has outcomes comparable to those of drug-eluting stents [3,4]. Statins are powerful cholesterol-lowering agents with pleiotropic effects. Statin therapy reduces cholesterol levels, induces the regression of coronary atherosclerosis, and stabilizes coronary plaques, improving patient outcomes [5]. Inhibitors of the renin-angiotensin system and beta-blockers also reduce atherosclerosis and its complications, such as myocardial infarction [6,7]. In our case, however, the use of these cardioprotective drugs was difficult because of the patient’s low blood pressure and low LDL cholesterol levels.
Several noninvasive cardiac tests have been used to evaluate CAD. However, a single test often has low diagnostic value. Further tests are frequently needed to improve diagnostic accuracy. Although CCTA has been considered an accurate diagnostic tool for detecting CAD, it provides no insight into the hemodynamic significance of luminal stenosis [8]. In our case, although CCTA revealed a non-calcified coronary plaque in the proximal LCX, the lesion did not receive attention due to the mild luminal narrowing. However, the exercise treadmill test result suggested myocardial ischemia. Our case shows the limitation of a single test and emphasizes the importance of additional tests, especially when the initial test results are inconclusive or do not match the clinical findings.
It is possible that vasospasm caused plaque rupture and thrombosis [9] in the LCX lesion. However, the clinical presentation of the chest pain was incompatible with variant angina in our case. Consequently, we did not perform a confirmatory provocation test during the initial ICA.
In conclusion, our case shows that AMI can occur after plaque rupture at a preexisting intermediate stenosis. Additional noninvasive diagnostic tests should be considered, especially in patients with ambiguous results of the initial test. In addition, FFR or IVUS evaluations during ICA, as well as subsequent optimal medical therapies, should be considered in patients with intermediate coronary stenosis.