Alemtuzumab을 이용한 이차성 순적혈구 빈혈의 치료
Successful Erythropoietin Therapy after Alemtuzumab and Cyclosporin a Treatment for Epoetin-Induced Pure Red Cell Aplasia
Article information
Trans Abstract
Pure red cell aplasia (PRCA) in adults is usually idiopathic, although some underlying conditions can cause PRCA. Immunosuppressive therapy (IST) is used to treat PRCA, but IST has side effects and may fail. The anti-CD52 monoclonal antibody alemtuzumab (ALM) was recently used to successfully treat therapy-resistant PRCA. We herein report successful treatment of secondary PRCA after erythropoietin therapy using ALM and cyclosporin A (CsA) in one patient. The total dose of ALM was 60 mg over 3 days (10, 20, and 30 mg, respectively) plus CsA for at least 6 months. The patient achieved a complete response 18 months after ALM-CsA treatment and his treatment could be changed to a different erythropoietin-stimulating agent. (Korean J Med 2013;85:214-217)
INTRODUCTION
Pure red cell aplasia (PRCA) is a severe normocytic, normochromic anemia involving a significant reduction in the number of reticulocytes in the peripheral blood and the absence of erythroid precursors in the bone marrow [1]. All other cell lineages are present and appear morphologically normal. Acquired PRCA is usually idiopathic, but it may rarely present secondary to erythropoietin (EPO)-stimulating agent therapy or allogeneic hematopoietic cell transplantation.
There have been several case reports and phase II studies of the effect of alemtuzumab (ALM), including our previous study of the effects of ALM and cyclosporin (CsA) treatment in 19 patients with bone marrow failure syndrome [2,3]. Our previous study enrolled one patient with idiopathic PRCA who achieved complete remission (CR). We herein report the effectiveness of ALM-CsA as a first-line therapy in a patient with secondary PRCA after EPO therapy. Management of anti-EPO antibody-mediated PRCA involves stopping recombinant EPO-stimulating agent (rESA) therapy and avoiding re-challenge to any alternative EPO preparations. However, a successful re-challenge was achieved in the patient with PRCA secondary to rESA in our report.
CASE REPORT
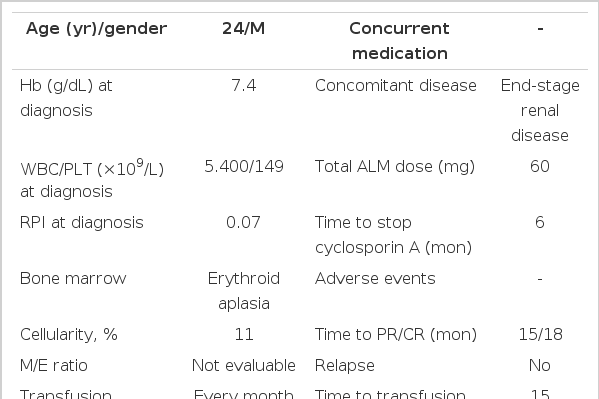
A 24-year-old male with end-stage renal disease due to chronic glomerulonephritis had been undergoing hemodialysis for 1 year. He was treated with an rESA (darbepoetin) for 2 years with sufficient efficacy. He had gradually developed anemia despite persistent EPO and intravenous iron therapy. It was believed that the rESA was no longer effective, and the patient was transferred to our hospital. His hemoglobin (Hb) level was 5.0 g/dL. The other blood results were as follows: mean corpuscular volume, 87.9 fl; mean corpuscular hemoglobin concentration, 33.4 g/dL; mean corpuscular hemoglobin, 29.4 pg; hematocrit, 0.142; and reticulocyte production index, 0.07. The white blood cell and platelet counts were normal. There was no evidence of iron or vitamin B12 deficiency. A peripheral blood smear showed anemia only, and bone marrow analysis revealed erythroid aplasia with 11% cellularity and no evaluable M/E ratio due to the absence of erythroid cells. His chromosome complement was 46, XY [15]. He had a history of EPO treatment for at least 3 weeks, the need for transfusion of about 1 unit/week to maintain a stable Hb level, reticulocytes of < 10 × 109/L, and no major decrease in leukocyte or platelet counts. Although the diagnostic criteria for epoetininduced PRCA include normal cellularity and < 5% erythroblasts on bone marrow aspiration with evidence of a maturation block, his bone marrow showed hypocellularity, almost erythroid aplasia, and normal maturation of other cell lineages; therefore, he was diagnosed with epoetin-induced PRCA [4]. We started ALM and CsA treatment. Prior to treatment, the patient was assessed for allergy to ALM by intravenous infusion of a test dose of 1 mg. He was premedicated 30 min before ALM infusion with acetaminophen (650 mg p.o.), pheniramine maleate (45.5 mg i.v.), and methylprednisolone (40 mg i.v.). He was treated with prophylactic sulfamethoxazole/trimethoprim (160 mg b.i.d. twice weekly) and acyclovir (200 mg b.i.d. daily) for the first 2 months. He received 60 mg of ALM over 3 consecutive days (10, 20, and 30 mg, respectively). The CsA dose was 1.5 mg/kg b.i.d. for 6 months, and adjustments were made depending on the plasma concentration and changes in renal function. CsA was started at the same time as ALM, and the plasma concentration of CsA was monitored twice weekly until a therapeutic level was achieved and maintained between 100 and 300 ng/mL. Thereafter, the CsA blood concentration was monitored once a month. The study protocol was approved by the Institutional Review Board of Ulsan University Hospital [2].
At follow-up, we were unable to measure Hb recovery because he had anemia due to end-stage renal disease. Therefore, a bone marrow study was performed. After 3 months, his bone marrow cellularity was 25%, and the M/E ratio had increased to 122. His bone marrow findings at 6 months after ALM were as follows: 18% cellularity and 47.2 M/E ratio. At that time, CsA therapy was stopped. After 7, 8, 9, 10, and 11 months of ALM, his Hb levels were 5.2, 8.3, 8.4, 6.4, and 7.8 g/dL, respectively. After 1 year of therapy, his bone marrow cellularity was 13% and his M/E ratio was 76. He had not achieved CR, but his bone marrow showed increased erythroid activity. We attempted treatment using a different rESA (epoetin 4,000 IU, 3 times weekly) that had not been administered previously. This led to increases in his Hb level to 10.2, 11.8, and 14.2 g/dL at 15, 16, and 18 months after ALM, respectively. He needed a transfusion every 2 weeks or 1 month until 15 months after ALM, when it could be discontinued. At that time, his bone marrow biopsy results showed 49% cellularity and an M/E ratio of 1.83; thus, he had achieved CR. He has maintained CR for 2 years 6 months (Fig. 1, Table 1).
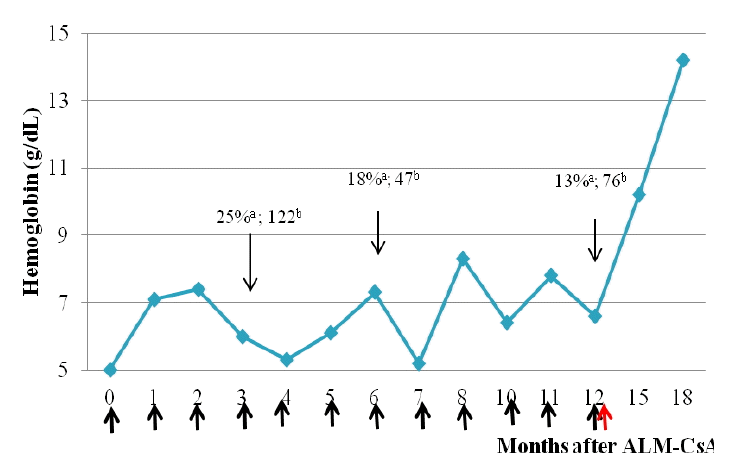
Changes in hemoglobin levels in patient with PRCA after erythropoietin use who received alemtuzumab Thick black arrows indicate packed red blood cell transfusion. Thick red arrow indicates use of recombinant erythropoietin-stimulating agent (rESA). ALM, alemtuzumab; CsA, cyclosporin. aBone marrow cellularity. bMyeloid/erythroid ratio in bone marrow.
DISCUSSION
There is no standard treatment for PRCA, but the most common treatment for idiopathic PRCA is immunosuppressive therapy (glucocorticoids, cyclophosphamide, or cyclosporin). Firstline treatments of idiopathic PRCA include glucocorticoids, glucocorticoids plus cyclophosphamide, and cyclosporine. Most patients respond to immunosuppressive treatment, but few prospective clinical trials using new agents have been performed with such subjects. For example, a study using the monoclonal anti-interleukin 2 receptor antibody daclizumab reported that 6 of 15 patients with PRCA achieved long-term packed red blood cell transfusion independence [5]. ALM is a monoclonal antibody that recognizes the CD52 antigen on the surface of T and B cells [6], and a number of studies recently reported the successful treatment of PRCA with ALM [2,3,7,8].
The acquired form of PRCA may occur with no apparent underlying cause or secondary to various underlying conditions such as autologous anti-EPO antibodies, ABO-incompatible allogeneic hematopoietic cell transplantation, or thymoma. This report describes a patient with secondary PRCA treated with ALM/CsA and in whom secondary PRCA developed after EPO therapy. There is no standard treatment for PRCA, and many studies, including our previous study [2,3,7,8], have reported successful responses to ALM. Therefore, we tried ALM as the first-line treatment in the present case.
Management of epoetin-induced PRCA involves stopping rESA therapy and avoiding re-challenge to any alternative EPO preparations. Re-challenge with rESA should be avoided because anti-EPO antibodies cross-react not only with the endogenous hormone, but also with all synthetic rESA molecules. Thus, an amnestic antibody response may occur, which may reduce the probability of the antibody returning to clinically normal levels [5,9,10]. The patient in our report was re-challenged with an alternative rESA that he had not received previously, and he has maintained CR for 24 months. Our case report is important because it demonstrates that a successful re-challenge can be achieved in patients with PRCA secondary to rESA.
This is the first report to demonstrate successful re-challenge of rESA in a patient with epoetin-induced PRCA by ALM-CsA treatment. The limitation of this report is that we could not prove that the anti-EPO antibody was the cause of the secondary PRCA. However, the clinical course of the patient was perfectly compatible with secondary PRCA caused by anti-EPO antibody.
In conclusion, the present patient with secondary PRCA showed CR to ALM-CsA. We conclude that ALM-CsA can be effective as a first-line therapy for epoetin-induced PRCA.