림프절에 발생한 골수외 고립 형질세포종
Extramedullary Solitary Plasmacytoma of the Lymph Nodes
Article information
Trans Abstract
Extramedullary plasmacytomas are uncommon malignant neoplasms that can occur in any organ. They arise most frequently from the upper aerodigestive tract. Lymph nodes are exceedingly rare sites of extramedullary plasmacytomas. Most extramedullary plasmacytomas of the lymph node arise in the cervical lymph nodes. Here, we report a case of an extramedullary plasmacytoma of the lymph node arising from a cervical lymph node. A 43-year-old male patient was admitted to our hospital and presented with a non-tender mass on the left side of his neck, which had been growing slowly for 1 month. The mass was excised. The pathology showed diffuse infiltration of immature plasma cells that were encapsulated by a layer of lymphoid cells, indicating an extramedullary plasmacytoma. He was treated with local radiation of the left cervical area. As of March 2009, he is doing well and shows no further evidence of the disease. (Korean J Med 2013;84:751-754)
INTRODUCTION
Most extramedullary plasmacytomas occur in the head and neck region and frequently arise in the upper aerodigestive tract. Primary lymph node plasmacytomas are rare malignant neoplasms [1-6]. They represent 2% of all extramedullary plasmacytomas, 0.5% of lymph node neoplasms, and only 0.08% of all malignant plasma cell neoplasms [4,7,8]. An extramedullary plasmacytoma of the lymph node is defined as a lymph node tumor comprising monoclonal proliferated plasma cells. However, it is necessary to exclude metastasis from multiple myeloma to diagnose a solitary extramedullary plasmacytoma [8]. Lim et al. [9] reported one case of lymph node plasmacytoma in Korea, in which multiple lymph nodes were involved. Here, we report a case of a single primary lymph node plasmacytoma that was differentially diagnosed by excluding multiple myeloma based on protein electrophoresis and bone marrow examination.
CASE REPORT
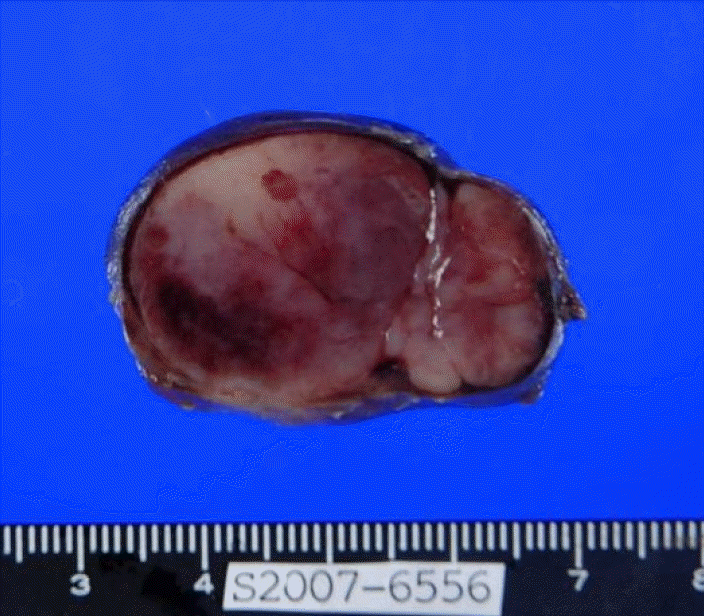
A 43-year-old male patient was admitted to our hospital and presented with a non-tender mass on the left side of his neck; this mass had been growing slowly for 1 month. He was asymptomatic and had no palpable masses anywhere in his body, except the neck. No other medical history or family history of cancer was evident. A solitary movable hard mass of the size of a Ping-Pong ball was palpable on the left cervical area. A computed tomography (CT) scan of the neck showed a 3 × 2.6 cm, well-defined, oval, homogeneously enhanced mass on the left side of the neck (Level II) and a 1.5 × 1 cm mass in the right thyroid (Fig. 1). The left cervical mass was excised and measured 3.8 × 2.6 cm (Fig. 2). The pathology showed diffuse infiltration of immature plasma cells encapsulated by a layer of lymphoid cells (Fig. 3A). The neoplastic nature of the plasma cell infiltrate was confirmed by immunohistochemical staining, which was negative for CD20 and positive for CD79a (Fig. 3B) expression. The staining of the specimen for immunoglobulin was positive for Lambda and negative for Kappa expression. The thyroid nodule proved to be thyroid hyperplasia.
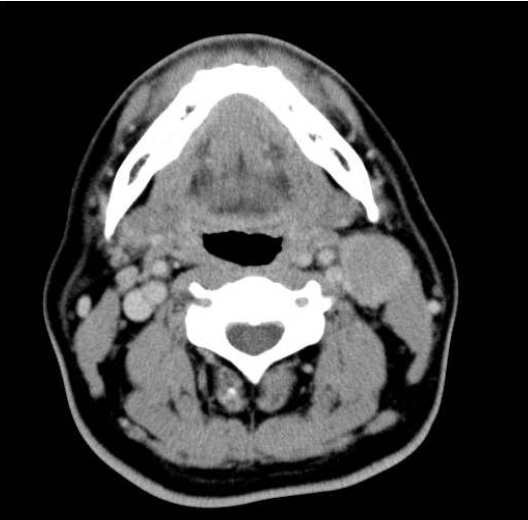
A computed tomography scan of the neck shows a well-defined mass on the left side of the neck (Level II).
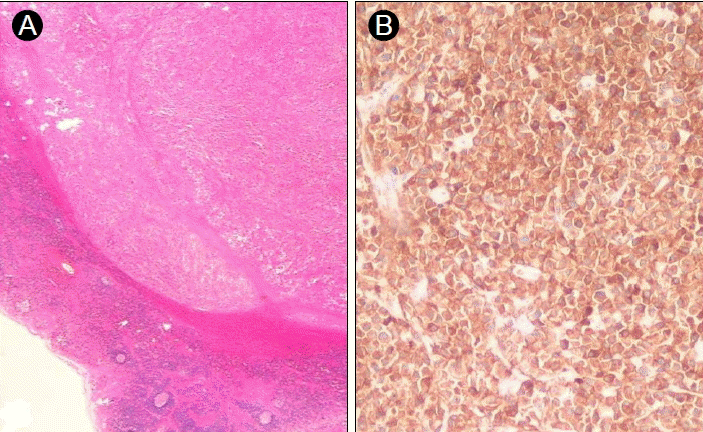
(A) Hematoxylin and eosin staining shows tumor cells encapsulated by a layer of lymphoid cells. (B) Immunohistochemical analysis reveals overexpression of the immunoglobulin-associated antigen CD79a.
We performed the following examinations to exclude multiple myeloma: hemoglobin content (14.6 g/dL), mean corpuscular volume(85.9 fL), white blood cell count (5.66 × 109/L), differential count (54.3% neutrophils, 37.5% lymphocytes, 4.6% monocytes, 3.2% eosinophils), platelet count (279 × 109/L), blood urea nitrogen(18 mg/dL), creatinine (1.2 mg/dL), calcium level (9.9 mg/dL), lactate dehydrogenase level (179 IU/L, normal value < 250 IU/L), and C-reactive protein level (0.1 mg/dL, normal value < 0.5 mg/dL). The serum transaminase levels were slightly increased (AST level, 37 IU/L; ALT level, 64 IU/L). The serum total protein level was 7.1 g/dL comprising 66.2% albumin (albumin level, 4.7 g/dL). The immunoglobulin levels were as follows: IgG, 910 mg/dL (normal range 700-1,600); IgA, 292 mg/dL (normal range 70-400), and IgM, 113 mg/dL (normal range 40-230). The serum β2-microglobulin level was within the normal limits. Serum protein electrophoresis(PEP) did not yield a monoclonal spike (M-spike) in the gamma globulin region. Additionally, urine PEP did not show an M-spike. Serum and urine immunoelectrophoresis (IEP) did not reveal monoclonal gammopathy. A skeletal survey revealed no evidence of bone lytic lesions. Bone marrow aspirates taken from the iliac crests were normal. Bone marrow biopsies showed no signs of multiple myeloma. Therefore, multiple myeloma was excluded, and a final diagnosis of a solitary extramedullary plasmacytoma of the cervical lymph node was made. The plasmacytoma was treated with radiotherapy (40 Gy in 20 fractions and 10 Gy in five fractions as a booster). Since then, the patient has been free of plasmacytomas.
DISCUSSION
Solitary plasmacytoma is classified as two types: solitary osseous plasmacytoma as an early form of myeloma, because of its high risk of subsequent dissemination, and extramedullary plasmacytoma. Primary extramedullary plasmacytomas are rare and have a lower incidence of progression to multiple myeloma or plasma cell leukemia than osseous plasmacytomas [7,8,10-12]. Extramedullary plasmacytomas occur most commonly in the upper aerodigestive tract, including the nasal cavity, sinuses, oropharynx, salivary glands, and larynx. Primary plasmacytomas of the lymph nodes are extremely rare, and few cases have been reported [1,2,5,6]. Most plasmacytomas of the lymph nodes appear to metastasize from multiple myeloma or from other extramedullary plasmacytomas. Primary plasmacytomas of the lymph nodes represent 2% of all extramedullary plasmacytomas [7,13]. These cases appear to be asymptomatic, except for localized swelling. They seem to have a better prognosis than other solitary extramedullary plasmacytomas that recur occasionally but rarely progress to multiple myelomas[14].
The diagnosis of a primary plasmacytoma of the lymph nodes is based on a localized, tumorous collection of monoclonal plasma cells without an associated component of malignant lymphoma, as shown by an immunophenotypic study [3]. Low-grade B-cell lymphoma, follicular lymphoma, monocytoid B-cell lymphoma, and lymphomas of the mucosa-associated lymphoid tissue (MALT lymphoma) may also show plasmacytic differentiation. In these tumors, the plasma cells are usually accompanied by many small neoplastic lymphocytes [3]. In our case, there was no evidence of nonplasmacytic neoplastic components. Immunoglobulin light and heavy chain expression can also distinguish primary plasmacytomas from malignant lymphomas [4]. Serum and urine PEP and IEP, as well as bone marrow examination, should be performed to exclude metastasis from multiple myeloma. In our case, serum PEP did not reveal an M-spike in the gamma region. Additionally, serum IEP revealed neither light nor heavy chain antisera. Bone marrow aspiration and biopsies were normal, indicating a primary solitary plasmacytoma.
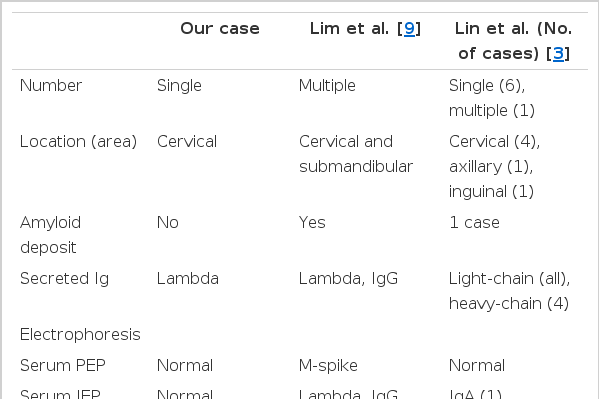
Some reports of primary lymph node plasmacytomas exist. In Korea, Lim et al. [9] reported one case of primary lymph node plasmacytoma. Compared with our patient, the case reported by Lim et al. had multiple plasmacytomas with an amyloid deposit [9]. Staining of a specimen for immunoglobulin and serum IEP from their case revealed positive expression of Lambda and IgG(Table 1). That patient was also treated with excision and radiotherapy. That patient survives to-date without evidence of recurrence or progression of the plasmacytomas to multiple myelomas. Lin et al. [3] reviewed the consultation files of the City of Hope National Medical Center (Duarte, CA, USA) and retrieved seven cases of primary lymph node plasmacytoma. According to their review, serum and urine PEP analysis of those patients did not show monoclonal protein expression at presentation. However, urine IEP analysis of one patient revealed the presence of monoclonal lambda light chains 7 years after diagnosis, and serum IEP analysis of another patient showed elevated monoclonal serum IgA. All patients showed immunoglobulin light-chain restriction. Four patients showed monoclonal heavy-chain expression, of whom three had IgG and one had IgM expression. All seven patients were treated with primary excision, and two received additional chemotherapy. All cases had an indolent clinical course, except for one patient in whom the plasmacytoma progressed slowly and who later showed an increased level of bone marrow plasma cells. None of these patients had recurrence or developed multiple myeloma.
It has been suggested that primary plasmacytomas of the lymph nodes may be clinically distinct from other extramedullary plasmacytomas because primary plasmacytomas have a more favorable short-term outcome and rarely progress to multiple myeloma [14]. Most reported cases responded well to excision, regardless of adjuvant chemotherapy or radiation therapy, and had no recurrence or progression. However, a minority of patients died of disseminated nodal disease. Some patients developed bone plasmacytomas, but no patient developed multiple myeloma [3]. Surgery is not necessary for diagnosis. Additionally, radical surgery is generally not indicated for cure because plasmacytomas are highly radiosensitive. Tumors less than 5 cm in diameter are suitable for local treatment with a radiation of a dose of 40 Gy in 20 fractions. Tumors greater than 5 cm in diameter have a higher local failure rate and may require a higher dose of radiation [2,5,6,11].