MTHFR 유전자 변이와 헤파린 유도 혈소판감소증이 동반된 환자에서의 성공적 생체 신장 이식 1예
Successful Living Donor Kidney Transplantation in a Patient with MTHFR Deficiency and Heparin-Induced Thrombocytopenia: A Case Report
Article information
Abstract
본 증례는 혈전 발생 위험도가 높은 MTHFR 유전자 변이와 헤파린유도 혈소판 감소증 두 질환을 동시에 가진 말기신부전 환자에서 신장 이식을 진행함에 있어, 수술 전후 혈전증 및 출혈을 최소화하는 개별화된 특수 항응고 치료 프로토콜을 적용하여 특이 합병증 없이 신장이식에 성공한 바, 이에 문헌고찰과 함께 보고하는 바이다.
Trans Abstract
Perioperative anticoagulation in a kidney transplant recipient with heparin-induced thrombocytopenia is challenging due to paradoxical hypercoagulability. A 49-year-old man with end-stage kidney disease and a previous stroke history was referred for living donor kidney transplantation. After the fifth dialysis session, the platelet count decreased to 10,000/μL, and heparin was immediately discontinued. Five days later, pulmonary thromboembolism with deep vein thrombosis was identified. Anti-heparin PF4 antibody, elevated serum homocysteine, and methylenetetrahydrofolate reductase gene mutations were discovered. Subsequent coronary angiography revealed three-vessel disease. Apixaban, folate, aspirin, and clopidogrel were administered and an inferior vena cava filter was implanted. Thereafter, coronary artery bypass graft surgery was performed using argatroban-bridging without complications. Kidney transplantation was performed 3 months later using the argatroban protocol. The graft kidney functioned well without any complications. In conclusion, we successfully conducted kidney transplantation in a thrombophilic patient with a methylenetetrahydrofolate reductase deficiency and heparin-induced thrombocytopenia by establishing an individualized perioperative anticoagulation protocol.
INTRODUCTION
Kidney transplantation (KT) is considered the best treatment option for patients with end-stage kidney disease (ESKD) [1]. Pre-transplant KT recipient candidates and perioperative management are usually evaluated based on the current KDIGO guidelines [1]. As the ESKD population has an increased risk of cardiovascular disease (CVD) compared to the general population, a pre-transplant CVD evaluation is crucial [1].
KT candidates require prompt intervention during the pre-transplant evaluation, such as percutaneous coronary intervention, and should take anticoagulants throughout the life course to delay KT and obviate the need for heparin-bridging during the perioperative period [1,2]. However, coagulation pathway disorders and hereditary diseases involving hypercoagulability, such as heparin-induced thrombocytopenia (HIT) or a methylenetetrahydrofolate reductase (MTHFR) mutation, may be encountered, which limits the use of heparin-bridging in this population. Furthermore, these conditions increase the risk of thrombotic events, which affect the outcomes of KT.
HIT is an iatrogenic disorder characterized by immune-mediated development of thrombocytopenia due to the administration of various forms of heparin [3]. It is caused by the formation of abnormal antibodies that activate platelets and paradoxically lead to a 30-fold increase in the risk of thrombosis [3,4].
Mutations in the genes encoding the MTHFR enzyme increase the total plasma homocysteine concentration, which is associated with premature arteriosclerosis and degeneration of the endothelium, and an increased risk of thromboembolism [5,6].
We report a case of successful living donor kidney transplantation (LDKT) in a patient with the HIT and MTHFR mutations, and a history of serious thrombotic events such as pulmonary thromboembolism (PTE), three-vessel disease, stroke, and deep vein thrombosis (DVT). A perioperative management protocol was designed, including anticoagulation with argatroban for HIT and folic acid replacement for MTHFR mutations.
CASE REPORT
A 49-year-old man with ESKD due to membranoproliferative glomerulonephritis was referred for LDKT. He had a 2-year history of stroke without neurological sequelae. The donor was his wife, and immunologic assessments showed negative cross-matching and negative donor-specific anti-HLA antibodies. Hemodialysis was initiated during the pre-transplant evaluation. After the first five hemodialysis sessions, his platelet count decreased from 416,000 to 10,000/μL; thus, heparin was immediately terminated and thrombocytopenia was evaluated, accompanied by platelet transfusion (Fig. 1). The patient complained of dyspnea 5 days later, and computed tomography revealed bilateral PTE and DVT (Fig. 2A, B). Apixaban was prescribed. Transthoracic echocardiography revealed a regional wall motion abnormality in the right coronary artery territory, which was not observed in an examination performed 2 weeks earlier. Further laboratory tests revealed positivity for the anti-heparin PF4 antibody, which confirmed HIT. The serum homocysteine level was 20.9 μmol/L (reference range: 3.0-15.0 μmol/L), and MTHFR gene mutations (A1298C hetero mutant [A/C] and C677T hetero mutant [C/T]) were discovered. Together, HIT and MTHFR mutations increase the risk of thromboembolic events and CVD.
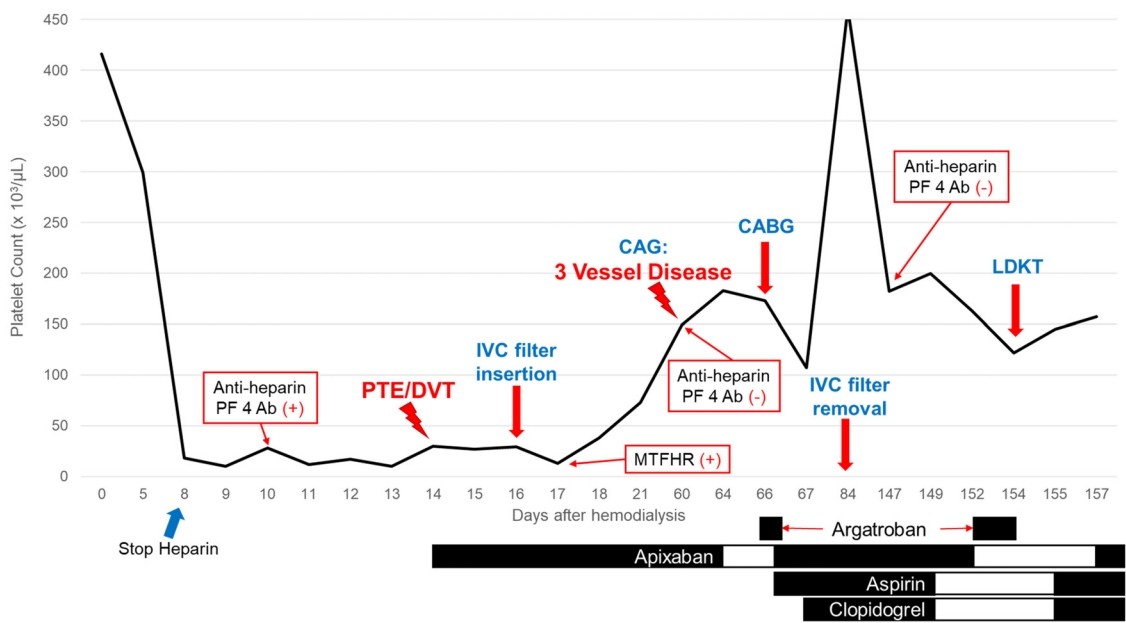
Summary of time-dependent changes in the patient’s platelet count. PF, platelet factor; PTE, pulmonary thromboembolism; DVT, deep vein thrombosis; IVC, inferior vena cava; MTHFR, methylenetetrahydrofolate reductase, CAG, coronary angiography; CABG, coronary artery bypass graft surgery; LDKT, living donor kidney transplantation.
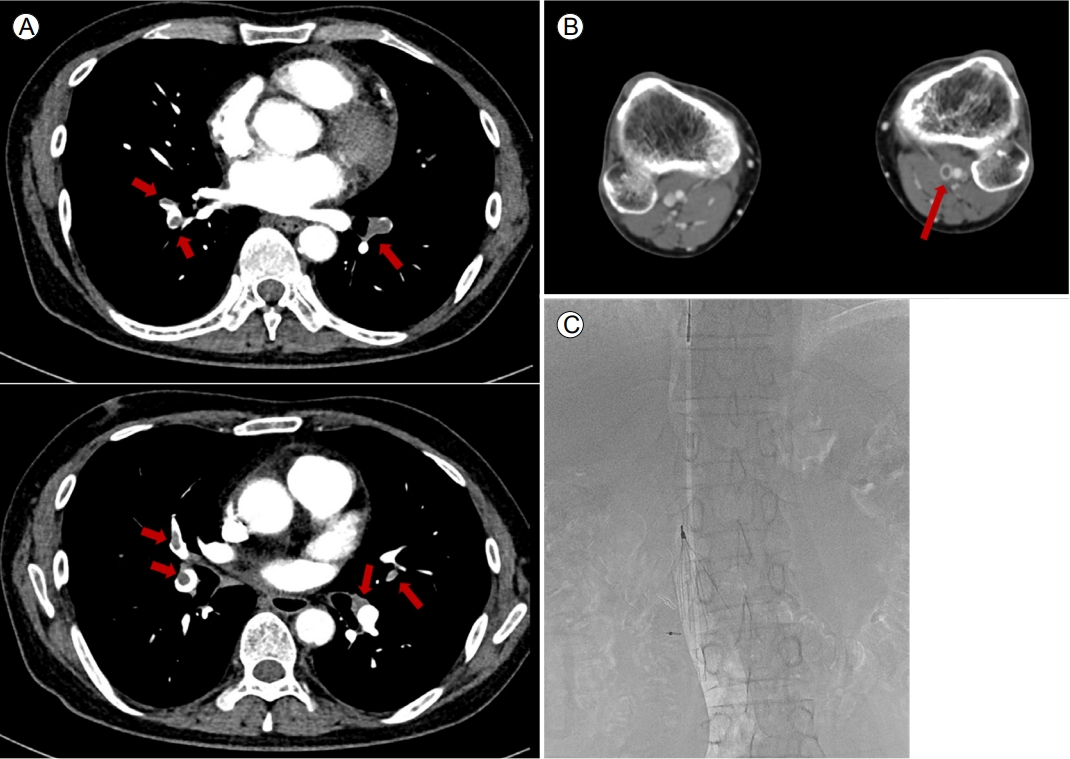
Computed tomography findings of (A) pulmonary thromboembolism and (B) deep vein thrombosis, and (C) placement of the inferior vena cava filter. (A) showed significant amount of pulmonary thromboembolism in both lungs (arrow). Small amount of deep vein thrombosis in the left popliteal vein (arrow) was presented in (B). Placement of inferior vena cava filter was noted on (C).
An inferior vena cava filter was immediately implanted to manage the PE and DVT (Fig. 2C). Consequently, heparin was avoided to prevent further thromboembolic events, and apixaban was maintained during hemodialysis for HIT, with folic acid replacement for the MTHFR mutations. Subsequent coronary angiography revealed three-vessel coronary artery disease (CAD) requiring coronary artery bypass graft (CABG) surgery (Fig. 3), and dual antiplatelet therapy with aspirin and clopidogrel was started. Although the anti-heparin PF4 antibody was not detected during the preoperative CABG evaluation, argatroban was replaced with perioperative heparin-bridging for CAD. The patient underwent CABG surgery without any thrombotic events or thrombocytopenia, and the KT was delayed for 3 months after the surgery.
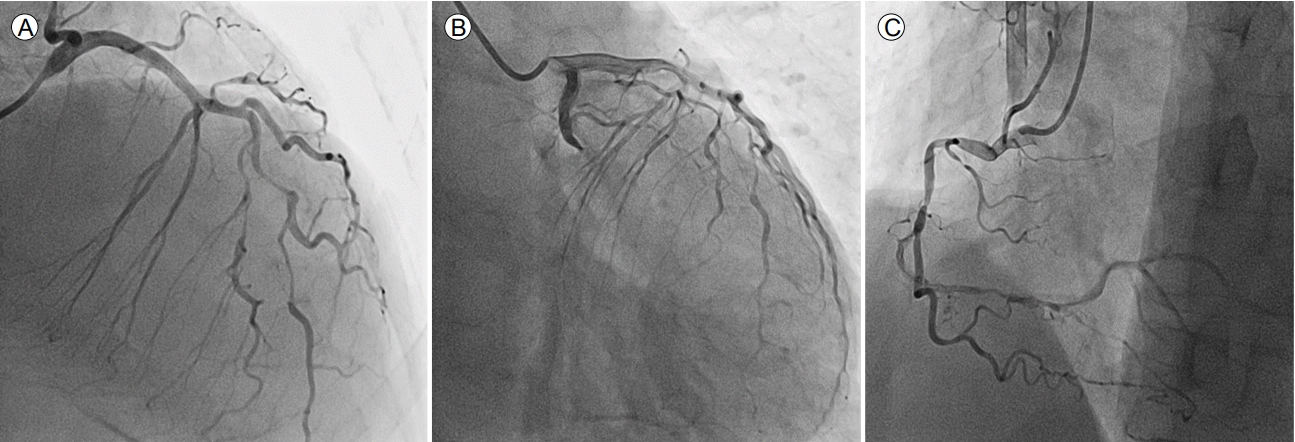
Coronary angiographic findings of the left LAD, LCX, and RCA. The mid-LAD exhibited 90% diffuse stenosis. The proximal LCX showed chronic total occlusion with collaterals from the diagonal branch. The proximal to mid-RCA had 80% diffuse stenosis and the mid-to-distal RCA was almost completely occluded with thrombus. LAD, anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery.
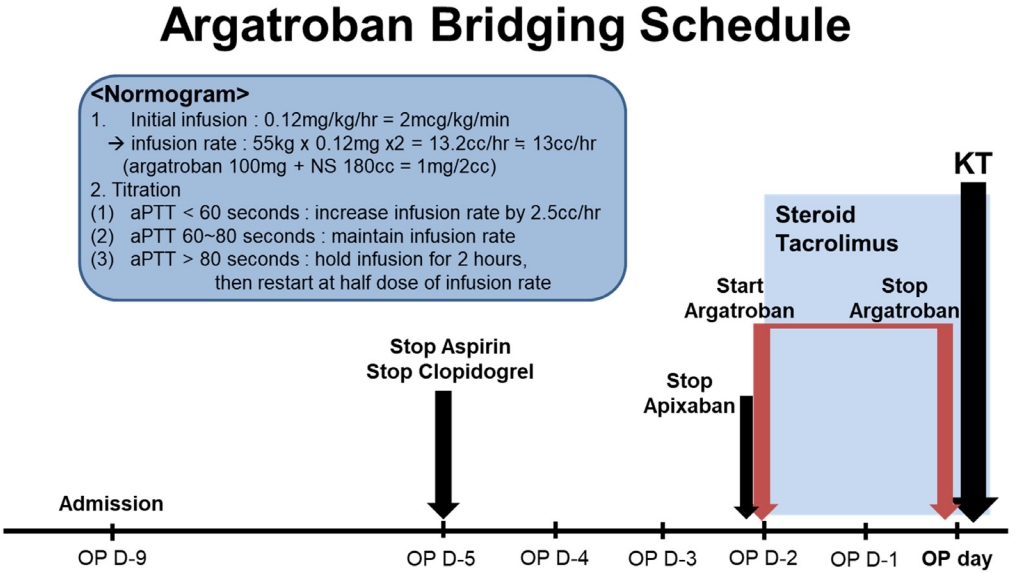
The patient was readmitted to the hospital for LDKT 3 months after the CABG surgery. Aspirin and clopidogrel were stopped 5 days before the KT, and apixaban was stopped, followed by the administration of an argatroban infusion, 2 days before the KT (Fig. 4). The argatroban infusion protocol was based on previous studies [4,7]. Basiliximab and immunosuppressants, such as corticosteroids, tacrolimus, and mycophenolate mofetil, were administered as scheduled, based on a routine protocol. The graft kidney functioned well immediately after transplantation, with no bleeding or thrombotic events. Aspirin and clopidogrel were resumed the day after the KT, and apixaban was added 3 days after the KT. Graft function has remained within the normal range, without any thromboembolic events, during the 2-year follow-up.
DISCUSSION
Graft and patient survival have improved over time in cases of deceased donor KT and LDKT, with 5-year LDKT graft survival rates of 85-91% being reported worldwide [8]. However, vascular complications such as bleeding and thrombosis are the leading causes of graft loss during the first year after transplantation, with allograft thrombosis accounting for 44% of early graft failures (within 30 days of transplantation) [8,9].
As KT is associated with a prothrombotic state, patients with either inherited or acquired thrombophilic disorders may have an increased risk of future thrombotic events [9]. Therefore, a priori detection of thrombophilic disorders via an appropriate strategy is necessary to prevent undesirable thrombotic complications. A laboratory evaluation of coagulopathy, as well as a complete family and medical history including thrombotic events before transplantation, may be helpful. Because it is difficult to identify patients at high risk of thrombosis, and given the lack of consensus recommendations for optimal prophylaxis during the preoperative period, in-depth consideration of the causes of previous thrombotic events, and a preoperative prophylaxis plan, are required to prevent catastrophic complications.
HIT is an immune-mediated drug reaction that leads to a 30-fold increase in the risk of thrombosis persisting until the platelet count recovers; this was observed in 5% of patients administered heparin [3,4,7]. Patients with ESKD who undergo hemodialysis are frequently exposed to heparin to prevent clotting of the dialyzer membrane, and a few patients experience severe thrombocytopenia suggestive of acute HIT [4]. Because half of acute HIT patients develop thromboembolism within 30 days, and the majority of events occur within 10 days of discontinuing heparin, minimum anticoagulation is recommended in cases of isolated HIT until the platelet count recovers [4,7]. Furthermore, acute HIT is also detected in patients undergoing their first dialysis at the pre-transplant evaluation, and the anti-heparin PF4 antibody typically persists for a median of 50-85 days after stopping heparin [4]. In this case, it is essential to develop a special anticoagulation protocol using alternatives such as argatroban (a direct thrombin inhibitor), which prevents serious thromboembolic complications during the peritransplant period.
A high plasma level of total homocysteine is a risk factor for various CVDs, and the relevance of hyperhomocysteinemia as a risk factor is even more pronounced in patients with chronic kidney disease due to their altered metabolism [9]. Mutations in MTHFR, particularly at nucleotide positions (MTHFR 677C>T or MTHFR 1298A>C), have been linked to an increased risk of thromboembolic events among ESRD patients [6,9]. These two mutations (double heterozygous MTHFR C667T and A1298C) were detected in the present case. To reduce the thromboembolic risk in such a population, it is essential to supplement with folic acid, vitamin B6, or vitamin B12. In particular, supplementation with these vitamins should continue for KT candidates with MTHFR mutations, to minimize the per-transplant thromboembolic risk.
The present patient had two major risk factors for thrombosis: MTHFR mutations with hyperhomocysteinemia and HIT. As a result, these two conditions may have contributed to his previous stroke; thus, he was classified as high risk for thrombosis. Therefore, a specific peritransplant anticoagulation protocol was established to minimize the risk of thromboembolism and perioperative bleeding. Direct thrombin inhibitors, such as argatroban and bivalirudin, have been approved as alternative non-heparin anticoagulants for patients with HIT. However, accumulating evidence suggests that argatroban is superior to other anticoagulants, and is safe and well-tolerated in patients with renal impairment [10]. Based on the current evidence, we established a KT protocol for a HIT patient using argatroban anticoagulation (Fig. 4), and performed a successful KT.
In conclusion, we successfully performed LDKT in a high-risk patient with thrombosis who had both hyperhomocysteinemia with MTHFR mutations and HIT by using an individualized special perioperative anticoagulation protocol. A priori detection of a thrombophilic disorder, and application of an adequate prevention strategy during the pre-transplant evaluation, are essential to prevent catastrophic thromboembolic complications.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science & ICT (2018M3A9E802151) and the Ministry of Education (2020R1I1A1A01072416).
AUTHOR CONTRIBUTIONS
Donghyuk Kang wrote the manuscript and analyzed the data; Eun Jeong Ko revised the manuscript; Donghyuk Kang, Hanbi Lee, Byung Ha Chung, Bum Soon Choi, Chul Woo Yang and Eun Jeong Ko designed the study and collected the data.
Acknowledgements
None.