CD19 양성 혼합표현형급성백혈병의 블리나투모맙 치료 1예
Treatment of CD19-Positive Mixed Phenotype Acute Leukemia with Blinatumomab
Article information
Trans Abstract
Mixed phenotype acute leukemia (MPAL) encompasses a rare group of acute leukemia with blasts expressing markers specific to several lineages, which accounts for 2-5% of all newly diagnosed cases of acute leukemia. At present, patients with MPAL are treated with acute lymphoblastic leukemia (ALL)-directed chemotherapy. However, the prognosis of MPAL, especially in cases of relapsed/refractory (R/R) disease, remains poor. Blinatumomab, a bispecific T cell-engaging antibody, has shown encouraging outcomes in R/R B cell ALL positive for CD19. Here, we report a patient with CD19+ MPAL who achieved complete remission after treatment with blinatumomab, which may be a therapeutic option for patients with relapsed CD19+ MPAL. To our knowledge, this is the first case report of MPAL treated with blinatumomab in Korea.
INTRODUCTION
Mixed phenotype acute leukemia (MPAL) encompasses a heterogeneous group of rare leukemias with a reported incidence of 2-5% of all newly diagnosed cases of acute leukemia [1]. With the limited data available, due to the rarity of disease, MPAL is currently treated with acute lymphoblastic leukemia (ALL)-directed chemotherapy if feasible, followed by allogeneic hematopoietic stem cell transplantation (HSCT) as consolidation therapy [2,3]. However, the prognosis of relapsed/refractory (R/R) MAPL is poor [1-3]. Blinatumomab is a bispecific T cell-engaging antibody that connects the CD3 T cell receptor to the CD19 antigen on B lymphocytes creating a cytolytic synapse, which mediates leukemic cell lysis [4]. Large clinical trials showed that blinatumomab has encouraging clinical efficacy with a favorable safety profile for the treatment of CD19+ R/R B cell ALL [4,5]. Here, we report a patient with relapsed CD19+ MPAL after transplantation who achieved complete remission(CR) with blinatumomab monotherapy.
CASE REPORT
A 44-year-old man with a history of hypertension presented with a 2-week history of petechiae and fatigue in August 2018. Physical examination revealed pale conjunctivae and scattered petechiae throughout the body. His complete blood count showed pancytopenia with a white blood cell (WBC) count of 3.65 × 109/L, hemoglobin level of 9.9 g/dL, platelet count of 21 × 109/L, and 18% circulating blasts, suggesting acute leukemia. Bone marrow (BM) examination showed hypercellularity (100%) with 80% blasts, and cytogenetic analysis revealed a complex karyotype (Fig. 1). Immunohistochemical staining for myeloperoxidase and B lymphocyte marker (CD19) and flow cytometry were performed (Figs. 2, 3). The results confirmed MPAL (B/myeloid) (Table 1). The patient initially received modified hyper-CVAD induction chemotherapy with cyclophosphamide, vincristine, doxorubicin (also known as adriamycin), and dexamethasone, but he did not achieve CR (10% blasts in BM and complex karyotype). After completing reinduction chemotherapy with cytarabine, mitoxantrone, and etoposide, he achieved his first CR in July 2018 with a BM blast count of 1% and 46,XY cytogenetics. As no HLA-matched sibling or unrelated donors were available, he received haploidentical stem cell transplantation from his sister with total body irradiation (800 cGy) plus a busulfan (6.4 mg/kg)/fludarabine (150 mg/m2)/anti-thymocyte globulin (5 mg/kg) regimen in October 2018. Immunosuppressive agents consisted of cyclosporin and short-course methotrexate. He displayed acute graft-versus-host disease (skin, grade 2), which resolved within the first month with systemic glucocorticoid. Peripheral blood chimerism was 100% donor at 1, 3, and 5 months after transplantation. At 6 months after transplantation, new-onset pancytopenia (WBC 3.4 × 109/L, hemoglobin 8.4 g/dL, and platelets 78 × 109/L) and circulating 5% blasts in peripheral blood prompted us to perform BM examination, which showed hypercellularity with 17% blasts expressing CD19 (Table 1) and complex karyotype. Due to the CD19 expression on blast cells, the patient received two cycles of blinatumomab as salvage treatment according to the manufacturer’s instructions, with no adverse side effects, such as neurological toxicity and cytokine release syndrome. After completion of two courses of blinatumomab therapy, each repeat BM aspiration/biopsy revealed normocellular BM with a blast count of 2% and 46,XX cytogenetics. After achieving second CR, he proceeded to a second allogeneic stem cell transplantation from a sibling donor (his brother) in July 2019. After the second transplantation, engraftment was achieved, and blood count remained stable. Unfortunately, however, the patient died suddenly of myocardial infarction due to heart failure 3 months after transplantation, without evidence of disease relapse.
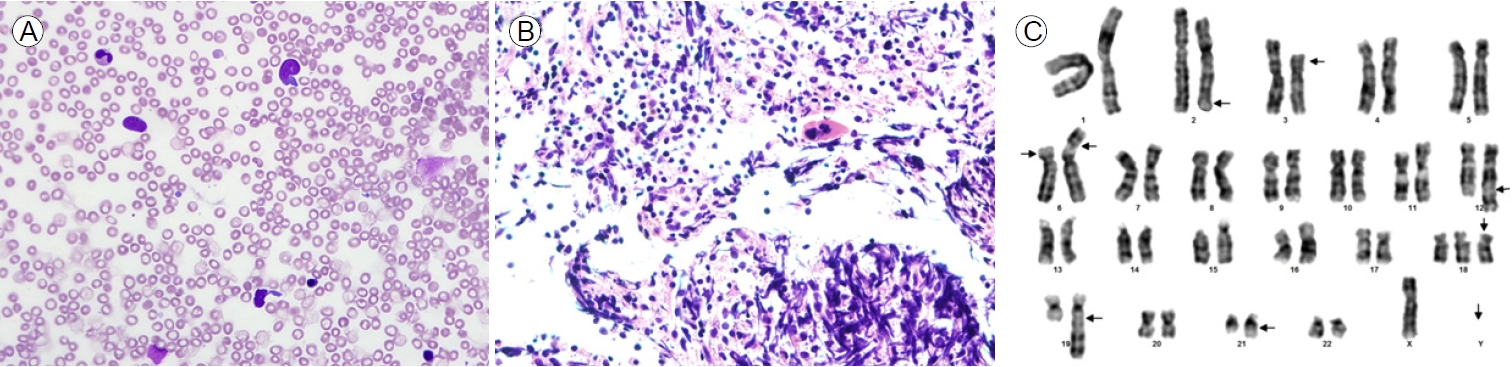
(A) Bone marrow aspirate smear showing predominantly blast cells (hematoxylin and eosin stain [H&E], ×400). (B) Bone marrow core biopsy section demonstrating markedly hypercellular bone marrow containing predominantly blast cells (H&E, ×400). (C) Giemsa banding karyogram: complex karyotype.
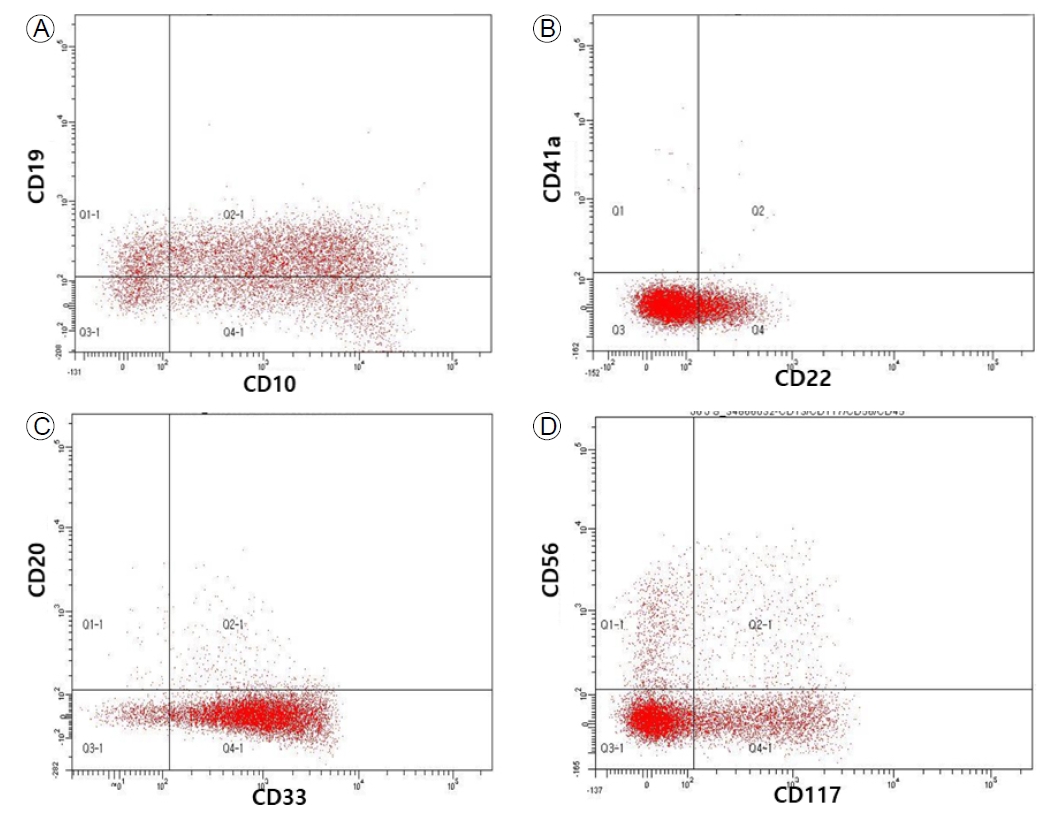
Flow cytometry showing the presence of leukemic cells expressing (A, B) B cell lineage markers, and (C, D) myeloid markers.
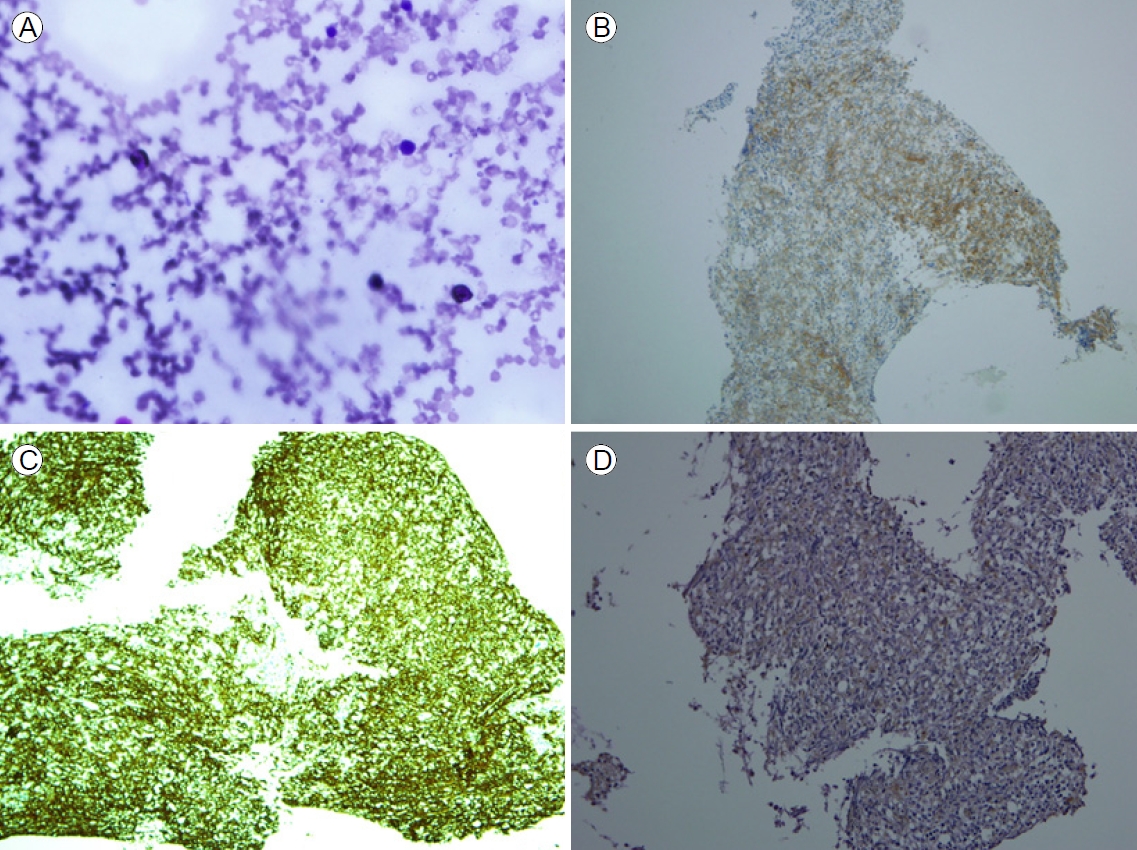
Immunohistochemical findings in mixed phenotype acute leukemia blast cells positive for (A) myeloperoxidase (hematoxylin and eosin stain [H&E], ×400), (B) CD19 (H&E, ×100), (C) CD20 (H&E, ×100), and (D) terminal deoxynucleotidyl transferase (H&E, ×200).
DISCUSSION
ALL-directed induction chemotherapy provides the best chance of achieving remission in patients with MPAL, but the response is usually transient, and relapse occurs frequently, leading to a poor survival rate [1]. At present, there are no standard of care options for patients with MPAL, with failure of initial induction chemotherapy or relapse after HSCT.
Blinatumomab, a first-in-class CD19/CD3 bispecific antibody, induces lysis of B cells by simultaneous binding to CD3-postive cytotoxic T cells and CD19-poistive B cells, and has shown promising efficacy in CD19+ B cell ALL in a salvage setting [4]. However, data are lacking regarding salvage therapy with blinatumomab in patients with CD19+ MPAL. There has been only one case report of two patients with MPAL receiving blinatumomab after conventional chemotherapy; El Chaer et al. [6] reported the efficacy of blinatumomab in the treatment of two patients with CD19+ MPAL, resulting in sustained minimal residual disease-negative remission in both patients. In this case, after relapse at 6 months after transplantation, our patient received blinatumomab as salvage therapy and achieved second CR with no treatment-related toxicity, proceeding to second HSCT. To our knowledge, this is the first report regarding blinatumomab treatment for MPAL in Korea.
The development of new therapeutic options for R/R MPAL, including targeted therapy, is an important area of research. As the CD19 surface antigen can be expressed on blasts at the time of relapse [1,2], blinatumomab may be a potential therapeutic option to prolong survival of patients with R/R MPAL expressing CD19, given that no effective salvage treatment exists for these patients.
In conclusion, we reported a case in which blinatumomab treatment provided a complete remission in a patient with CD19+ MPAL, even though the disease relapsed early after HSCT. Blinatumomab may be useful for treatment of patients with CD19+ MPAL, with an insufficient response to conventional treatment, and therefore warrants further evaluation in prospective clinical trials.
