소장 폐색의 위석을 금속 스텐트 삽입 및 압박하여 제거
Removal of a Small Bowel Bezoar Causing Bowel Obstruction via Mechanical Compression after Metal Stent Placement
Article information
Trans Abstract
Small bowel obstructions (SBOs) that develop for various reasons often require prompt medical treatment. Migration of a gastric bezoar (indigestible foreign material that has accumulated in the stomach) is a rare cause of SBO. Treatment of a symptomatic SBO caused by a bezoar requires a multidisciplinary approach that considers the patient’s physical status and comorbidities and the bezoar volume, location, and pathology. Although surgery is the treatment of choice, endoscopic treatments such as fragmentation and retrieval may serve as alternatives. We present the first case of resolution of a large phytobezoar via mechanical compression after covered metal stent insertion, followed by stent retrieval, in a patient with a symptomatic SBO that persisted even after two sessions of push-endoscopic fragmentation.
INTRODUCTION
A phytobezoar (the most common type of bezoar) is a trapped indigestible mass in the gastrointestinal (GI) tract that includes fruit and vegetable fibers, cellulose, hemicellulose, lignin, and tannins [1]. The stomach is the most common source of bezoar formation [1]. Gastric bezoars are discovered during fewer than 0.5% of all upper GI endoscopies [1]. Medical conditions (impaired stomach motility), stomach surgeries (vagotomy or a gastric bypass), mixed connective tissue disease, and hypothyroidism are associated with bezoar formation [2]. The most common causes of mechanical small bowel obstruction (SBO) are postoperative adhesions and hernias. An SBO is potentially life-threatening and often requires surgery. An SBO caused by a bezoar is uncommon (2-4% of all SBOs) [3]. SBO is usually caused by migration of a gastric bezoar. The most common site of obstruction is the terminal ileum 100 cm proximal to the ileocecal valve [1]. A persimmon phytobezoar can develop in a patient without a history of GI surgery because high concentrations of tannin can polymerize in the presence of gastric acid, serving as a nucleus for bezoar formation [3]. Although an SBO caused by a trapped bezoar commonly requires surgical intervention, endoscopic fragmentation of a bezoar in the distal ileum is a possible alternative [4]. Here, we report a case of an SBO caused by a large persimmon phytobezoar that was resolved via mechanical compression using a covered metal stent inserted into the distal ileum, followed by stent retrieval, after failed endoscopic fragmentation despite two sessions of argon plasma coagulation.
CASE REPORT
A 76-year-old female who was almost bedridden for several years for a reason that was not clear to us presented with abdominal pain and vomiting 10 days in duration. Her history included 5 mg olanzapine daily to treat a long-standing psychotic disorder, no abdominal surgery, and no disease that might cause an electrolyte disturbance. Her initial vital signs were stable. Physical examination revealed diffuse abdominal pain. Initial laboratory findings revealed a white blood cell count of 19,300/μL, hemoglobin level of 12.3 g/dL, blood urea nitrogen/creatinine level of 22/1.3 mg/dL, and C-reactive protein level of 89 mg/L. Esophagogastroduodenoscopy revealed multiple active gastric ulcers in the antrum. Abdominal computed tomography (CT) revealed an obstructive ileus, a markedly dilated small bowel with enhanced wall-thickening but without a specific obstructive lesion (the pattern was suggestive of feces-like material), and a distal lesion located just before a severely angulated area lying 50-70 cm from the ileocecal valve (Fig. 1A, B). Regarding dietary habits, the patient reported that she had consumed a high-fiber diet over the past several months. Bowel preparation was not possible prior to colonoscopy because the patient could not tolerate oral liquids; two enemas were performed prior to examination. Colonoscopy using an intermediate-length (130 cm) colonoscope (CF-H260 AL, Olympus Optical Co. Ltd., Tokyo, Japan) revealed no specific lesion. Capsule endoscopy for further detection of small bowel abnormalities (such as a hidden malignancy) was performed after we explained that the capsule might be retained and that an obstructive tumor might require surgical treatment; the patient provided informed consent. Capsule endoscopy revealed that the capsule had failed to pass beyond the pylorus despite ingestion 12 hours previously, strongly suggestive of probable future capsule retention in the small intestine. Capsule retention in the middle abdominal area was suggestive of a small bowel lesion (which was also evident on a plain abdominal X-ray). Further evaluation of the distal ileum within 70-80 cm from the ileocecal valve using a standard 160-cm-long colonoscope (CF-H260 AL; Olympus Optical Co., Ltd.) to perform push enteroscopy up to 70 cm from the ileocecal valve revealed a large phytobezoar, an SBO, and surrounding severe mucosal edema with a thick white exudate (Fig. 1C). Fragmentation via argon plasma coagulation (APC) and retrieval of the fragmented bezoar using a basket and polypectomy snare were attempted. However, treatment for 30 minutes was inadequate, as residual bezoars were evident (Fig. 1D). Her symptoms did not improve despite her attempts to drink 500-1,000 mL Coca-Cola daily over 3 days. Although surgical treatment was recommended to the patient and her family, the patient declined such treatment and requested further endoscopic treatment. We performed additional APC fragmentation and retrieved some bezoar fragments using various accessories. However, there were no improvements in the mechanical ileus evident on X-rays nor in the abdominal pain or distension. We then performed (after obtaining informed consent) mechanical compression of the partially fragmented phytobezoar in the distal ileum via insertion of a covered metal stent, as an attempt to relieve the SBO. Follow-up push enteroscopy using a colonoscope revealed large amounts of retained fragmented phytobezoars 70 cm from the ileocecal valve. We used APC to puncture the center of the bezoar (blue arrow in Fig. 2A) and inserted a 22-mm-diameter, 8-cm-long covered metal stent (Bona Stent, Standard Sci-Tech Inc., Seoul, Korea) to mechanically compress the softened, partially fragmented bezoar (Fig. 2B). Follow-up abdominal CT performed on the following day revealed a well-positioned metal stent (long white arrow in the distal ileum and the retained capsule (short white arrow) (Fig. 2C). The patient’s abdominal pain and distension gradually improved. We encouraged a daily intake of 300-500 mL Coca-Cola. The stent was removed 5 days later because no evidence of malignancy was found. The third follow-up abdominal CT revealed improvement of the ileus and the capsule (short white arrow in Fig. 2D) in the right colon (Fig. 2D). Moderate wall-thickening with possible fibrotic changes in the region of the earlier distal ileal lesion was also observed (arrow of Fig. 2E, F). The patient was discharged 3 days after stent removal. She has been doing well for the past 16 months.
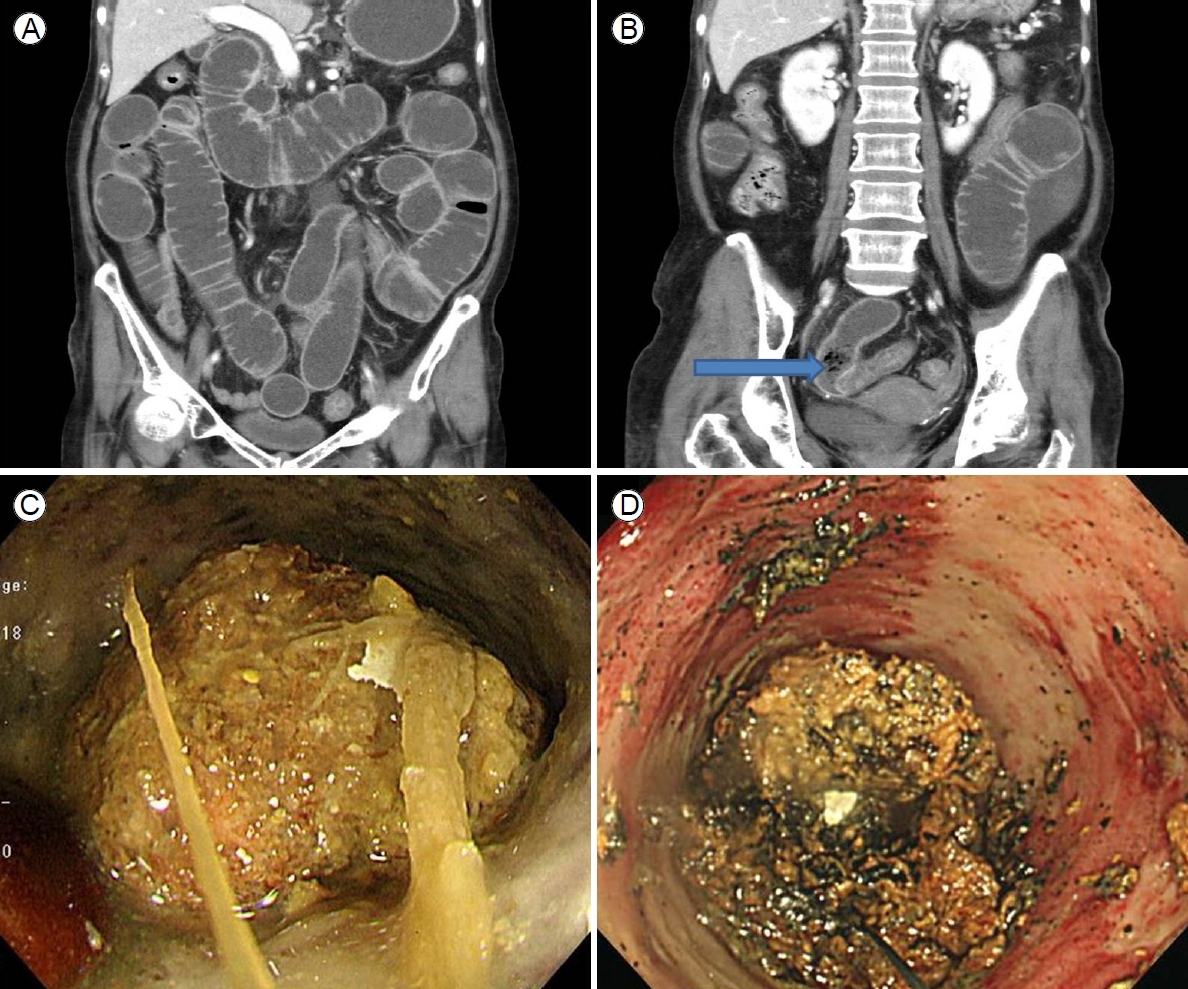
(A, B) Abdominal computed tomography revealed an obstructive ileus, a markedly dilated small bowel with enhanced wall-thickening but without a specific obstructive lesion (the pattern was suggestive of feces-like material; arrow of B), and a distal lesion lying just before a severely angulated area ranging from 50 to 70 cm from the ileocecal valve. (C) Colonoscopy that penetrated to 70 cm from the ileocecal valve revealed a large phytobezoar, a small bowel obstruction, and surrounding severe mucosal edema with a thick white exudate. (D) Bezoar fragmentation was achieved via argon plasma coagulation.

Follow-up colonoscopic push endoscopy revealed partially fragmented phytobezoars. (A) The center of the bezoar was punctured (arrow) via argon plasma coagulation. (B) A covered metal stent was inserted to mechanically compress the softened residual bezoar. (C) Follow-up abdominal computed tomography (CT) revealed a well-positioned metal stent (long white arrow) in the distal ileum and the capsule (short white arrow). (D) The third follow-up CT revealed improvement of the ileus and the capsule (short white arrow) in the right colon. (E, F) Moderate wall-thickening with possible fibrotic changes in the earlier distal ileal lesion was also observed (arrow).
DISCUSSION
The bezoar was a phytobezoar caused by a high-fiber diet. Migration of the phytobezoar into the small bowel, followed by SBO, was suggested by the endoscopic findings; the multiple active ulcers in the antrum were probably caused by prior formation of gastric phytobezoars. The bezoar was located 70 cm from the ileocecal valve, and the tract was narrow and intestinal motility slow. Extensive water absorption contributes to bezoar formation [1]. Also, our patient exhibited a unique (severe) anatomical angulation just distal to the location of the bezoar. Gastric bezoars may be asymptomatic; however, they may also be associated with GI symptoms such as nausea, vomiting, delayed gastric emptying, and weight loss. Small bowel bezoars usually present with abdominal pain and distension, vomiting, and constipation [5,6].
Bezoars can be classified into the following four types according to the oral source: phytobezoars, trichobezoars, pharmacobezoars, and lactobezoars [7]. Imaging studies, including plain abdominal radiography, barium studies, ultrasonography, CT, and small bowel series, are often used to diagnose GI bezoars. Endoscopy remains the diagnostic method of choice [7]. However, distinguishing an SBO caused by an adhesion from that caused by a bezoar is not easy, especially in patients who have undergone previous abdominal surgery, even when various imaging modalities are employed. Abdominal ultrasonography is non-invasive and has a diagnostic accuracy of nearly 88% [8]. Abdominal CT can diagnose an SBO caused by a bezoar, with a diagnostic accuracy of 73-95%; CT can also reveal the cause of bowel obstruction, multiple bezoars (if present), and complications such as bowel perforation [9]. Capsule endoscopy is preferred when evaluating small bowel disease. Although rare, capsule retention concerns clinicians; however, it is impossible to guarantee that the capsule will not be retained [10]. Although the mechanical ileus lacking a definitive obstructive lesion, according to CT, in our patient might be considered a contraindication to capsule endoscopy, we nonetheless performed capsule endoscopy to identify the cause of the SBO. Capsule retention was informative.
A bezoar must be removed and recurrence prevented. The management strategies include lavage/dissolution, fragmentation, and/or retrieval. Lavage/dissolution requires ingestion of acetylcysteine, papain, cellulose, or Coca-Cola; however, some adverse events have been reported [7]. An SBO caused by a phytobezoar that migrated after initial successful dissolution by Coca-Cola required surgery [11]. Coca-Cola ingestion was ineffective in our patient. Many fragmentation/retrieval methods are available, of which endoscopic fragmentation combined with polypectomy snares or baskets, laser treatments, and APC are preferred [1,7,12,13]. Although endoscopic treatment is optimal, surgery should be considered for patients who fail endoscopic therapy or who exhibit complications (significant bleeding, a major obstruction, or a perforation). Several reports on successful endoscopic removal of small bowel bezoars have appeared [4]. A colonoscopic approach is possible unless the bezoar is located distal to the distal ileum. Such an approach was possible in our patient. After two unsuccessful APC fragmentations/retrievals, we delivered mechanical compression by inserting a covered metal stent into the softened, partially fragmented phytobezoars after central puncture via APC. This is a novel approach that resolved the SBO. The capsule moved into the colon, and the stent was removed because the patient lacked a malignancy.
Insertion of a covered metal stent is useful when treating various GI tract leaks and fistulae [14,15] and also when seeking to control post-procedural bleeding, bleeding from malignant tumors, or GI perforations with strictures [16-18]. The stent mechanically compresses the underlying membrane. We hypothesized that insertion of a covered metal stent into the center of a mass of softened, partially fragmented bezoars will compress the mass and thus maintain small bowel luminal patency. The SBO vanished using this approach. However, more cases are required.
In summary, if a patient exhibits an SBO caused by a phytobezoar, insertion of a covered metal stent followed by stent removal within 7 days mobilizes refractory, incomplete, partially fragmented phytobezoars. This may valuably obviate the need for surgery.