INTRODUCTION
The incidence of tuberculosis (TB) in South Korea has steadily decreased in recent years; however, the overall incidence of multidrug-resistant tuberculosis (MDR-TB) remains steady, with 700-900 new cases per year [1]. Bedaquiline (Bdq) and delamanid (Dlm) are new drugs that can be used to treat MDR-TB in cases where standard treatment regimens are ineffective. The approved treatment duration for Bdq and Dlm is limited to just 24 weeks based on phase 2 clinical trial data [2]. In addition, because of a major concern about QT prolongation, the combination of Bdq and Dlm is only recommended for extraordinary situations in which the benefits outweigh the risks [3]. The number of patients using this new drug combination for MDR-TB is increasing [4]; however, no cases have been reported in which both drugs have been used concurrently for more than 24 weeks in Korea. Here, we present the case of a patient with pulmonary MDR-TB who was successfully treated with a combination of Bdq and Dlm for 48 weeks.
CASE REPORT
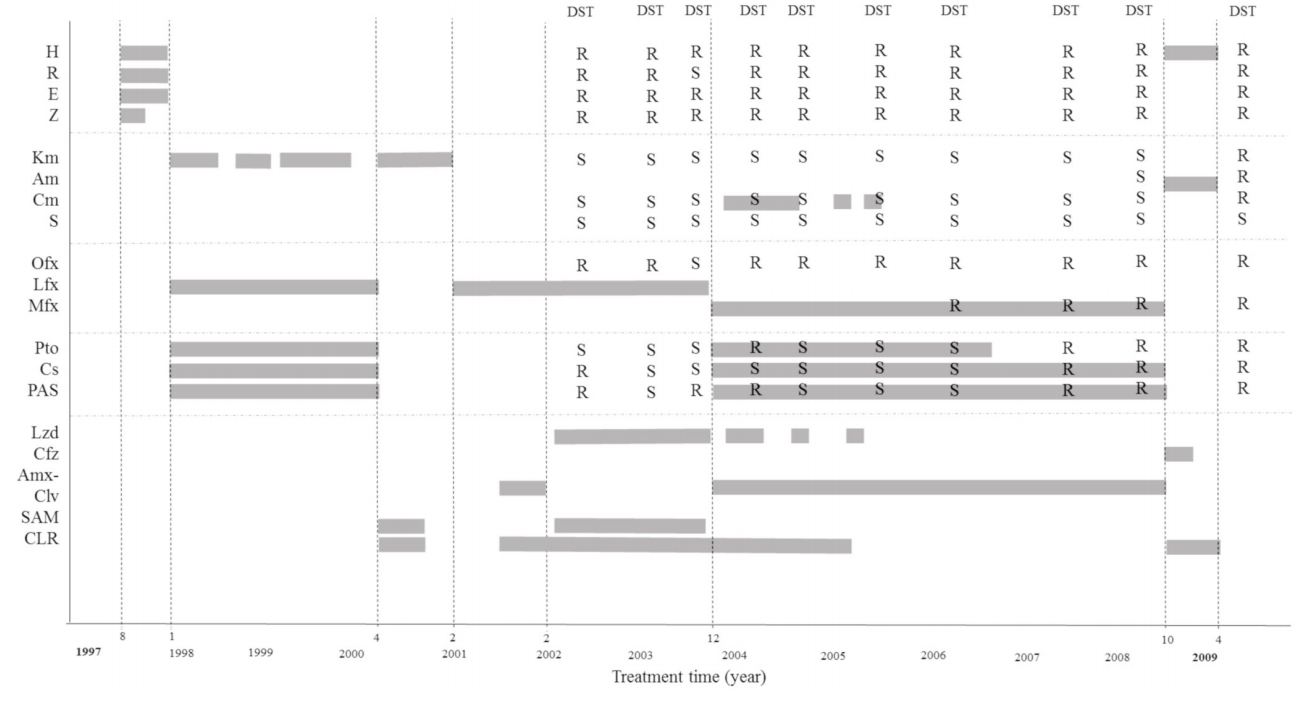
A 45-year-old woman was admitted to an isolation room for treatment of pulmonary MDR-TB. The patient was first diagnosed with MDR-TB 20 years prior, having been unsuccessfully treated with several conventional drug regimens at another hospital. She was transferred to our hospital in February 2004, after which treatment was reinitiated using a multidrug regimen including linezolid (Lzd) (Fig. 1). Sputum acid-fast bacilli (AFB) stain/culture showed negative conversion immediately after treatment initiation but reverted to positivity after discontinuation of Lzd due to an adverse event. The treatment was stopped in April 2009 due to a shortage of effective drugs. The last drug susceptibility test (DST) revealed extensively drug-resistant TB. Thus the patient was observed without treatment until 2017. In 2017, concurrent use of both Bdq and Dlm was approved by the Korean Centers for Disease Control and Prevention (KCDC) for treatment of MDR-TB. Additional approval was granted by the Institutional Review Board of Asan Medical Center for cases where prolonged duration of treatment was deemed necessary.
The patient was a non-smoker with no history of other systemic diseases. Physical examination revealed a pulse rate of 125 beats per minute, blood pressure of 120/83 mmHg, respiratory rate of 26 breaths per minute, body temperature of 36.6Ōäā, and oxygen saturation of 95% based on pulse oximetry. Her body weight was 58.4 kg. The patient was fully conscious without any neurological deficits. Bronchial breath sounds without crackles were dominant at the bilateral upper lung. The examination of other systems was essentially normal. Laboratory results were as follows: hemoglobin concentration of 10.0 g/dL, white blood cell count of 10,000/mm3, neutrophil differential of 77.7%, lymphocytes 12.6%, and monocytes 6.7%, and a platelet count of 267,000/mm3. Serum levels of electrolytes, renal function, and liver function tests were normal. A screening test for human immunodeficiency virus antibody using an electrochemiluminescence immunoassay was negative.
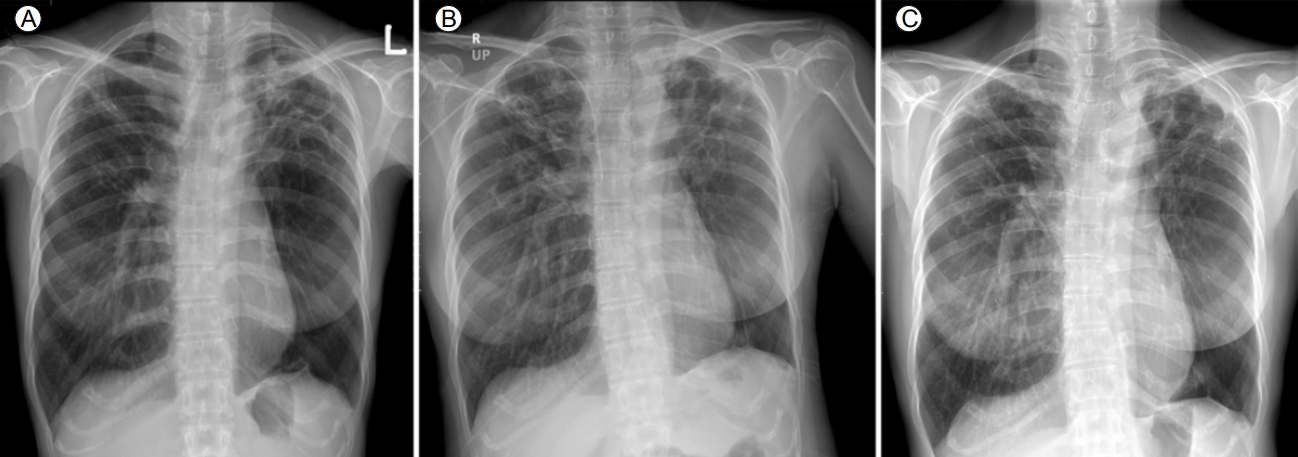
A chest radiograph revealed multiple cavitary lesions with tiny nodules, which were particularly dominant in both upper lung zones (Fig. 2). The QT duration in electrocardiograms corrected using FridericiaŌĆÖs formula (QTcF) was 404.7 ms. Sputum cultures of Mycobacterium tuberculous had been persistently positive since 2004. Drug susceptibility testing (DST) was conducted by the Korea Institute of Tuberculosis (Osong, Korea) with Lowenstein-Jensen media using an absolute concentration method. Testing revealed resistance to isoniazid, rifampin, ethambutol, ofloxacin, rifabutin, moxifloxacin, levofloxacin, and pyrazinamide but susceptibility to second-line injectable drugs (amikacin, kanamycin, and capreomycin). Critical concentrations were as follows: isoniazid, 0.2 ╬╝g/mL; rifampin, 40.0 ╬╝g/mL; kanamycin, 30.0 ╬╝g/mL; capreomycin, 40.0 ╬╝g/mL; amikacin, 30.0 ╬╝g/mL; ofloxacin, 4.0 ╬╝g/mL; levofloxacin, 2.0 ╬╝g/mL; and moxifloxacin, 2.0 ╬╝g/mL [5].
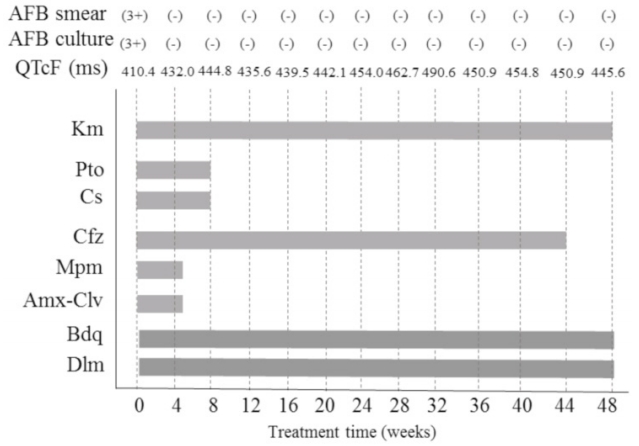
Treatment with both Bdq and Dlm was initiated on August 23, 2017 (Fig. 3). The dose for each drug was as follows: Dlm, 100 mg twice a day; Bdq, 400 mg once a day for 2 weeks followed by 200 mg three times a week; clofazimine (Cfz), 100 mg once a day; prothionamide (Pto), 375 mg twice a day; cycloserine (Cs), 250 and 500 mg twice a day; kanamycin (Km), 15 mg/kg once a day; meropenem (Mpm), 1,000 mg three times a day; and amoxicillin/clavulanate (Amx-Clv), 625 mg two times a day. Directly observed therapy was performed during hospitalization. During treatment, laboratory tests (including serum levels of potassium, sodium, magnesium, calcium, and electrocardiogram results) were regularly monitored. Sputum smears of AFB and cultures were repeated monthly. The patient continued to take the medication without any major adverse events, including QT prolongation. On the 38th day after treatment initiation, she was discharged to her home, where she maintained the same regimen with the exception of Mpm and Clv. A nurse monitored the patientŌĆÖs condition once a week by telephone.
The patient was followed up monthly at an outpatient clinic. Two drugs were discontinued: Cs due to hand tremors and Pto due to nausea. On the 41st week after treatment initiation, Cfz was stopped due to skin hyperpigmentation (cosmetic reasons). Bdq, Dlm, and Km were continued until the 48th week. A full breakdown of the treatment regimen is shown in Figure 3. The peak QTcF (491 ms) was observed on May 31, 2018; no clinically significant cardiac adverse events developed during treatment. Sputum culture results became negative 30 days after starting treatment (September 21, 2017), with negative conversion sustained until the completion of treatment. The treatment was successfully completed on July 27, 2018, with a clinical assessment indicating complete clearance of the TB infection.
DISCUSSION
Bedaquiline and delamanid were recently approved for the treatment of MDR-TB. A treatment duration of 24 weeks was established based on phase 2 clinical trial data, although the combined use these two drugs is typically not recommended because it may exaggerate QT prolongation.
In Korea, the treatment of MDR-TB using two new drugs, Bdq and Dlm, was approved by the TB expert review committee of the KCDC [3]. Despite approval, the prolonged use of these drugs beyond 24 weeks is prohibited. The concurrent use of both new drugs is permitted only when effective treatment is impossible using conventional drugs or only one of the new drugs. This case report represents the first incident in which a patient with pulmonary MDR-TB was treated concurrently with Bdq and Dlm beyond 24 weeks in Korea. These observations are consistent with recent studies conducted outside of Korea, including several reports describing patients treated with one or both drugs for more than 24 weeks, as necessary [6-8]. No serious adverse events were reported in these cases. In this study, prolonged and concurrent use of the two new drugs resulted in successful treatment. In addition, despite the safety concerns, clinically significant QT prolongation was not observed. We expect that the concurrent and prolonged use of Bdq and Dlm will be expanded to include a larger number of difficult-to-treat MDR-TB patients.
To prevent the development of resistance and to maximize the efficacy of the new drugs, it is recommended that treatment regimens include additional companion drugs. In our case, Cfz was included due to the short period of use in the patientŌĆÖs past treatment. Pto and Cs were included based on the patientŌĆÖs recent phenotypic DST results, although the treatment had failed in the past. Previously, the bacilli were resistant to Km and Amk but had recently shown to be susceptible to them; thus, we included Km in the patientŌĆÖs treatment regimen as well. Although Mpm/Amx-Clv had never been used in the past, the DST result was not available for Mpm/Amx-Clv and their treatment duration was only 40 days. These findings suggest that the successful treatment outcome in our study was mainly induced by the new drugs, Bdq and Dlm.
Clinical trials are currently being conducted with new regimens, including Bdq or Dlm, to treat MDR-TB for 6-9 months [9,10]. However, the duration of conventional regimens that include the new drugs is generally 18-24 months [3]. In our case, the total treatment duration was 48 weeks for three reasons: sputum culture conversion was achieved early, after 1 month of treatment; few effective drugs remained after completing 48 weeks of treatment with the two new drugs; and the cost of the drugs was too expensive for the patient to pay herself (the expenses for the initial 24 weeks were supported by the government, but additional costs needed to be paid by the patient). The appropriateness of the treatment duration will be determined after long-term follow up to screen for TB recurrence.
It is likely that this patient infected several people with MDR-TB bacilli prior to starting Bdq and Dlm combination therapy in August 2017. It is unknown how well the patient adhered to recommendations to remain largely isolated at home. Non-compliance would not have been surprising, as the new drugs are thought to substantially reduce the risk of infecting others. Although the risks of concurrent use of two new drugs and over 24 weeks of use are not well known, in this case, we believe that the benefits of using both drugs outweighed the risk of drug toxicity.
For our patient, DST was performed at the time of treatment failure in 2009, revealing resistance to second-line injectable drugs and fluoroquinolones. However, over the following 9 years in which the patient was not treated, the bacilli became susceptible again to the second-line injectable drugs.
In conclusion, prolonged and concurrent treatment with Bdq and Dlm under regular surveillance is a possible treatment option for patients with intractable MDR-TB without severe adverse events. Subsequent studies of concurrent and extended treatment beyond 24 weeks with the two new drugs are urgently needed.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print






