진행 담낭암에서 포트시스템을 이용한 간동맥항암제 주입 요법
Hepatic Arterial Infusion Chemotherapy Using the Port System in Advanced Gallbladder Cancer
Article information
Trans Abstract
Gallbladder (GB) cancer is relatively rare and has a poor prognosis, with a median survival time of less than 3 months. It is resistant to chemotherapy. Therefore, the role of systemic chemotherapy is limited. However, administering the anticancer agent directly into the hepatic artery can result in a higher drug concentration in the cancer tissue. In this paper, we report a case of advanced GB cancer treated with hepatic arterial infusion chemotherapy (HAIC) using the port system. The patient received six cycles of HAIC with 5-fluorouracil (750 mg/m2) and cisplatin (25 mg/m2); each cycle lasted for 4 days every month. The tumor showed objective response during HAIC, and the patient survived for 15 months from the first therapy. HAIC using the port system might be a promising therapeutic modality for treating locally advanced GB cancer.
INTRODUCTION
Gallbladder (GB) carcinoma is relatively rare and has a poor prognosis, with a median survival time of less than 3 months [1]. Surgical resection offers the only chance for a cure; however, only 10% of patients present with early-stage disease [2]. Most patients with GB cancer have advanced disease at presentation because of the propensity for lymph node metastasis, direct invasion of the liver, and seeding of the peritoneal cavity. In a review of 227 patients with nonresectable GB cancer who were treated by systemic chemotherapy, the overall response rate was 11.9% [3].
Administering the anticancer agent directly into the hepatic artery results in a higher drug concentration in the cancer tissue compared to systemic administration. Therefore, hepatic arterial infusion chemotherapy (HAIC) might be a reasonable approach for treating locally advanced cancer confined to the liver [4].
Previously we reported good tumor response to HAIC using the port system in advanced hepatocellular carcinoma (HCC) [5]. Although HAIC is an effective therapeutic modality for treating advanced HCC, to the best of our knowledge it has not been reported for treating inoperable GB cancer. In addition, only a few cases have been reported in the English literature [6,7].
In this paper, we report an objective tumor response during HAIC in a GB cancer patient with massive hepatic invasion and metastasis.
CASE REPORT
A 62-year-old man was admitted to Yeungnam University Hospital for right upper quadrant abdominal discomfort lasting for a month. Several years prior to admission he had been diagnosed with chronic hepatitis B but had not undergone further evaluation or treatment. Abdominal computed tomography (CT) showed a GB fundal mass with subhepatic extension, multiple liver metastasis and extensive subhepatic and para-aortic lymphadenopathies (Fig. 1A and 1B). Angiography also showed a massive tumor stain located in both lobes of the liver (Fig. 1C). Laboratory data were as follows: white blood cells 9,190 k/uL, hemoglobin 13.1 g/dL, platelet count 252 K/uL, total bilirubin 0.9 mg/dL, aspartate aminotransferase 30 U/L, alanine aminotransferase 19 U/L, albumin 4.15 g/dL, prothrombin time 102%, α-fetoprotein 8 ng/mL, protein induced by vitamin K absence or antagonist II 267 U/L, CA19-9 44.8 U/mL, HBsAg positive, HBeAg negative, HBeAb positive, HBV DNA < 2,000 copies/mL.

Computed tomogram and angiogram show gallbladder fundal mass invading the liver parenchyma and massive hepatic metastasis (A-C). The chemoport was inserted in the right thigh for hepatic arterial infusion chemotherapy (D).
A port was inserted for hepatic arterial infusion chemotherapy (Fig. 1D). The patient was treated with 5-fluorouracil (FU) and cisplatin. 5-FU 750 mg/m2 was diluted with 5% dextrose water and 200 mL was administered over 2 hours using a portable infusion pump from day 1 to day 4. Cisplatin 25 mg/m2 was diluted with normal saline and 200 mL was given over 1 h using an intra-arterial catheter from day 1 to day 4. The chemotherapy was repeated every 4 weeks.
The treatment was relatively tolerable, although the patient complained of anorexia and nausea. After the first two cycles of chemotherapy, an objective tumor response was observed. Therefore, the chemotherapy continued through six cycles (Fig. 2). During therapy, the liver mass decreased markedly and lymphadenopathies were not extended. After the sixth cycle, further chemotherapy was stopped because of fears of systemic adverse effects. The port was removed and the patient received supportive care. Two months after the last chemotherapy cycle (10 months after the first therapy), the patient was readmitted for sudden abdominal pain caused by colonic obstruction. Positron emission tomography and CT showed multiple metastases in both supraclavicular nodes, abdominal lymph nodes, left lobe of the liver, and right colon. Biopsy from a neck node on the left side showed metastatic carcinoma suggesting biliary origin (Fig. 3). The patient received two cycles of palliative systemic chemotherapy (5-FU, 1 g/m2 from day 1 to day 5; cisplatin, 100 mg/m2 on day 2) and radiotherapy (28 fractions, total dose of 5,040 cGy). During therapy, the colonic obstructive symptoms were relieved slightly. However, the patient died of massive gastrointestinal bleeding 15 months after the first therapy cycle. The clinical course of the patient is summarized in Table 1.

After six cycles of chemotherapy, most tumors in the right lobe disappeared and the tumor in the left lobe regressed from 7 cm before therapy to 3 cm in diameter (A), but direct colonic invasion (arrow) was still noted (B).
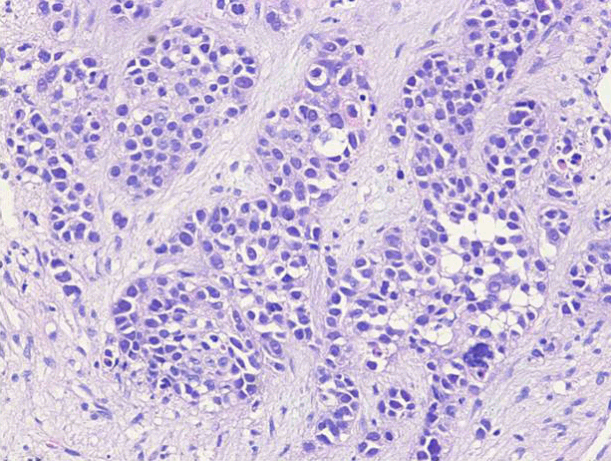
Biopsy of the neck soft tissue was performed on the left side. Anaplastic round to polygonal cells proliferated in an arrangement of solid nests (hematoxylin and eosin staining, ×200). Immunohistochemical staining revealed tumor cells positive for cytokeratin 7 and 19. These cells were negative for both hepatocyte common antigen and alfa-fetoprotein (data not shown).
DISCUSSION
Although some beneficial effects of chemotherapy have been reported in several clinical trials, the use of systemic chemotherapy for treating inoperable advanced biliary cancers is generally limited because of the known chemoresistance of this type of cancer. In a review of 227 patients with nonresectable GB cancer who were treated by systemic chemotherapy, the overall response rate was 11.9% [3]. Furthermore, there were no standard chemotherapeutic regimens and few randomized controlled studies [2].
HCC is also resistant to chemotherapy. Therefore, systemic chemotherapy is not generally accepted as a treatment option. However, HAIC using the port system has shown favorable clinical outcomes, including a good tumor response and prolonged median survival [4,5]. Currently, HAIC is a treatment option for advanced HCC in Korea [8].
HAIC administered via an injection port delivers repeated arterial infusions of anticancer agents that are exposed mainly to the liver; systemic adverse effects are consequently minimized. Therefore, HAIC might be a reasonable approach to treating patients with locally advanced cancer confined to the liver. However, there are no standard therapeutic regimens.
In terms of HAIC administration, 5-FU is the most frequently used anticancer drug in advanced HCC. The mechanism of action of the enhanced antitumor effects of 5-FU has been clarified. 5-FU is metabolized in cells to 5-fluoro-2’-deoxyuridine 5’-monophosphate (5’FdUMP), which inhibits thymidylate synthetase. The following misincorporation can lead to single-strand breaks, and RNA can aberrantly incorporate FUMP. Combination therapy with biochemical modulators, such as cisplatin, interferon-alfa, methotrexate, or leucovorin rather than 5-FU monotherapy, amplifies the antitumor effects [4,9]. Cisplatin can amplify the effects of 5-FU by biochemical modulation as well as its antitumor effects [9].
In this case, we used a combination of high-dose 5-FU and cisplatin based on our long-term clinical experience with advanced HCC. This regimen is safe and tolerable, and it elicits a good tumor response (57.1%) and improves survival among responders, with a median survival of 14 months in advanced HCC with portal vein tumor thrombosis [5]. Some patients show complete remission or receive curative resection after downstaging [4,5]. Chatni et al. [10] also reported that this regimen showed acceptable toxicity.
Based on these observations and rationale, we treated our 62-year-old male GB cancer patient with HAIC using high-dose 5-FU plus cisplatin. The tumor size decreased objectively and the patient’s quality of life improved during therapy, although the cancer progressed after the cessation of the last therapy cycle.
Taken together, these findings indicate that HAIC using the port system might be a promising therapeutic modality for treating locally advanced GB cancer.