 |
 |
| Korean J Med > Volume 91(3); 2016 > Article |
|
Abstract
Cardiac allograft vasculopathy is one of the most important causes of poor long-term survival after heart transplantation. The condition tends to be diffuse, usually affecting the mid-to-distal portions of the coronary artery. Reperfusion therapy is ineffective. Everolimus, an inhibitor of proliferation signaling, has been reported to prevent development of the condition; however, the efficacy thereof has not yet been fully accepted. The only definitive treatment for cardiac allograft vasculopathy is retransplantation. Herein, we describe the case of a 15-year-old boy who underwent heart retransplantation because of rapidly progressive cardiac allograft vasculopathy.
Heart transplantation (HTx) is a good option for selected patients with heart failure (HF) refractory to medical management. Outcomes after HTx have improved significantly in recent years: the 1-year survival rate now approaches 90% [1]. The major cause of long-term mortality in HTx patients is cardiac allograft vasculopathy (CAV), characterized by diffuse concentric narrowing of the coronary arteries. No successful treatment for CAV has yet been described; cardiac retransplantation remains the definitive effective treatment [2]. Here, we report on a pathologically confirmed case of CAV treated via heart retransplantation. We also review the diagnosis and treatment of CAV.
A 15-year-old boy underwent HTx four years prior to treat end-stage HF attributable to dilated cardiomyopathy. The donor was a 17-year-old boy who died of a subdural hemorrhage incurred in a motorcycle accident. One year after transplantation, the patient experienced an episode of acute cellular rejection (grade IA) and underwent methylprednisolone pulse therapy (1 g/day for 3 days) followed by prescription of cyclosporine and mycophenolate mofetil (MMF). However, he was otherwise asymptomatic and his functional status was New York Heart Association (NYHA) class I.
One year ago, he reported the gradual development of dyspnea and chest discomfort and was admitted for evaluation. Laboratory tests, electrocardiography (ECG), echocardiography, and computed tomographic coronary angiography (CTCAG) were performed. No laboratory test, the ECG, or echocardiography yielded significant findings. CTCAG revealed stenosis of the circumflex arterial ostium; diffuse stenosis of the mid-to-distal left anterior descending artery (LAD); and tight stenosis of the posterolateral branch of the right coronary artery (RCA) (Fig. 1). Invasive coronary angiography (CAG) was performed; this also revealed diffuse narrowing of the coronary arteries, with multiple stenoses. In summary, we observed focal stenosis (75%) of the mid-segment and total occlusion of the distal segment of the LAD; focal stenosis (75%) of the proximal left circumflex coronary artery (LCx); and subtotal occlusion of the postero-lateral branch of the RCA. The focal lesions in the proximal LCx and postero-lateral RCA branches were treated via ballooning followed by stenting with Xience Xpedition everolimus-eluting-stents (Fig. 2). We suspected CAV (ISHLT CAV2; thus of moderate grade) [3], characterized by the presence of multiple sequential lesions together with diffuse narrowing of the coronary arteries. The patient was on antihypertensive medication, but exhibited no other risk factors for CAV (e.g., no dyslipidemia or any history of cytomegalovirus [CMV] infection). He was discharged on aspirin, clopidogrel, statin, and antihypertensive drugs. He was also prescribed the immunosuppressives cyclosporine and everolimus, which replaced his MMF.
Eight months later, he was re-admitted with worsening dyspnea and chest discomfort. Echocardiography revealed moderate pericardial effusion, considered to be an adverse effect of everolimus; everolimus was discontinued. One follow-up CAG, the previous stent in the proximal LCx was patent, but the distal LCx and first-diagonal branch exhibited diffuse stenosis (95%). CAG also revealed total occlusion of the distal LAD. Ballooning was performed at the first diagonal branch, the distal LAD, and the distal LCx (Fig. 3). However, despite treatment, his condition progressively deteriorated; he experienced shortness of breath, NYHA class IV, nausea, loss of appetite, and oliguria. Follow-up echocardiography revealed decreased pericardial effusion and normal left ventricular systolic function. Laboratory analyses revealed newly developed hyponatremia (sodium 120 mmol/L) and N-terminal prohormone of brain natriuretic peptide elevation (to 2100 pg/mL). These findings suggested that rapidly progressive (and severe) CAV was the cause of symptoms and signs refractory to medical management. We thus decided to perform cardiac retransplantation. The patient was listed as a cardiac recipient candidate in the Korean Network for Organ Sharing (KONOS), and cardiac retransplantation was performed 2 months later. Pathological examination of the extracted heart revealed multiple diffuse luminal stenosis with intimal fibrosis, consistent with severe CAV (Fig. 4). After surgery, his symptoms improved and he was discharged on a statin, MMF, and tacrolimus. Currently, the patient is being followed-up and reports no complications.
CAV is a disease unique to HTx recipients. CAV is a diffuse pan-arterial process, characterized by intimal proliferation as a result of cumulative endothelial injuries followed by luminal stenosis of epicardial vessels in later stages. This complex process is inflicted by both immunological responses and non-immunological factors including older donor age, ischemia-reperfusion injury, CMV infection, hypertension, and dyslipidemia [2]. CAV continues to limit the long-term survival of HTx patients. Indeed, 5 years after transplantation, more than 30% of HTx recipients exhibit significant CAV, and 7% die or require retransplantation because of CAV-related events [4].
CAG is used for CAV screening and surveillance and is actually often employed to diagnose CAV. However, CAG cannot visualize the arterial wall per se, allowing only measurements of luminal diameter [3]. Thus, it is difficult to detect early-stage CAV. Intravascular ultrasound (IVUS) potentially yields accurate luminal diameters, and can quantify intimal thickening and vessel wall morphology. The superior sensitivity of IVUS has recently caused this imaging modality to become the test of choice for detection of early-stage CAV [5]. Recent technological advances in CTCAG now allow assessment of the arterial wall and the distal small vessels. Good-quality images can be obtained using small amounts of contrast agents and radiation; the modality is now clinically useful [3,5]. In our present case, we did not use IVUS for diagnosis. CTCAG was initially performed and invasive CAG was repeated during follow-up.
CAV treatments have been disappointing. Percutaneous intervention may palliate patients with focal stenosis of proximal or middle vessels, but the restenosis rates are high. Statins and calcium channel blockers exhibit some protective effects, but do not eliminate the problems [2]. As cellular proliferation is a key feature of CAV, anti-proliferative agents (including everolimus and sirolimus) should reduce the severity and incidence of the condition. However, proliferation signal inhibitors (PSIs) do not reverse CAV; they only prevent progression. Moreover, PSIs are often discontinued because of their significant adverse effects, including generalized edema, delayed wound-healing, oral ulcers, gastrointestinal problems, and other toxicities [6-8]. Heart retransplantation remains the only viable definitive treatment for end-stage CAV. An annual report of the International Society for Heart and Lung Transplantation found that survival after retransplantation was 70% at 1 year and 38% at 10 years [1]. These figures are distinctly poorer than those after de-novo HTx. Retransplantation remains controversial; the operation has a poorer prognosis than de novo transplantation. However, the survival rates after retransplantation in CAV cases are distinctly better than those after retransplantation to treat acute cardiac rejection with graft failure, and the outcomes have clearly improved over time. Some investigators have suggested that, in CAV patients undergoing retransplantation, the clinical outcomes are identical to those of patients undergoing primary transplantation [9,10]. Together, the observations suggest that CAV may be an appropriate indicator for heart retransplantation.
In conclusion, we describe the case of a 15-year-old boy who underwent heart retransplantation because of suspected CAV based on both CTCAG and conventional CAG, and eventually confirmed by pathological examination. Our case exhibited a typical clinical course in terms of both radiological images and pathological findings. The CAV was successfully treated via heart retransplantation.
REFERENCES
1. Lund LH, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first official adult heart transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant 2014;33:996‚Äď1008.


3. Mehra MR, Crespo-Leiro MG, Dipchand A, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant 2010;29:717‚Äď727.


4. Costanzo MR, Naftel DC, Pritzker MR, et al. Heart transplant coronary artery disease detected by coronary angiography: a multiinstitutional study of preoperative donor and recipient risk factors. Cardiac Transplant Research Database. J Heart Lung Transplant 1998;17:744‚Äď753.

5. Pollack A, Nazif T, Mancini D, Weisz G. Detection and imaging of cardiac allograft vasculopathy. JACC Cardiovasc Imaging 2013;6:613‚Äď623.


6. Eisen HJ, Tuzcu EM, Dorent R, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med 2003;349:847‚Äď858.


7. Mancini D, Pinney S, Burkhoff D, et al. Use of rapamycin slows progression of cardiac transplantation vasculopathy. Circulation 2003;108:48‚Äď53.


8. Gonzalez-Vilchez F, Vazquez de Prada JA, Almenar L, et al. Withdrawal of proliferation signal inhibitors due to adverse events in the maintenance phase of heart transplantation. J Heart Lung Transplant 2012;31:288‚Äď295.


Coronary computed tomography angiographic images. The posterolateral branch of the right coronary artery (RCA) exhibits tight stenosis (A, black arrowhead). The ostium of the left circumflex artery (LCx) exhibits subtotal occlusion (B, black arrowhead). The mid-to-distal region of the left anterior descending artery (LAD) exhibits diffuse luminal narrowing (C).
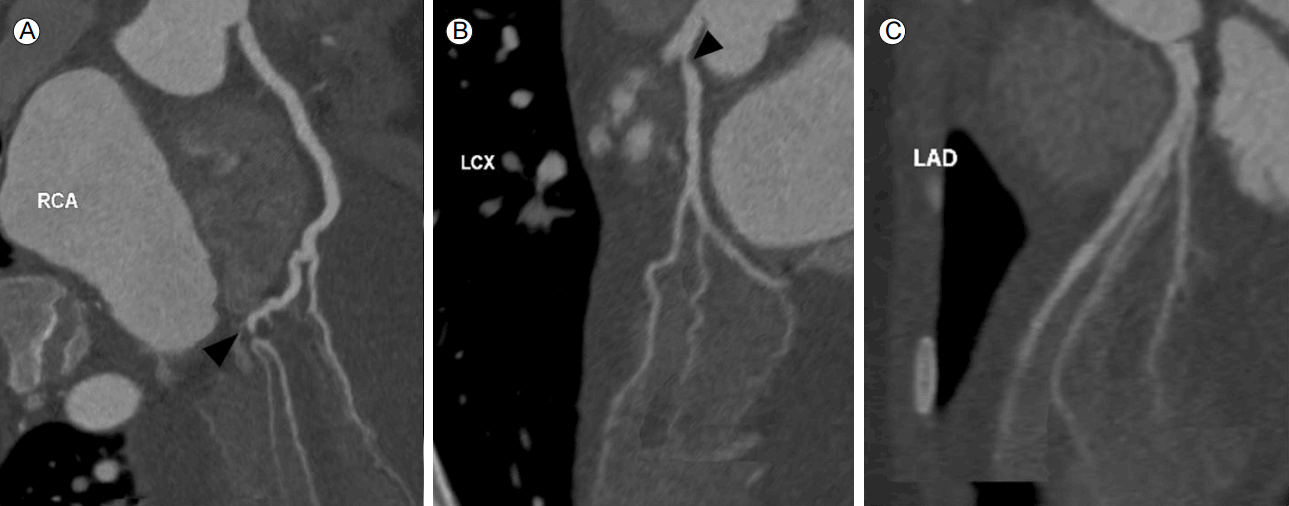
Figure 1.
Coronary angiographic images. A and B (RAO caudal views) and C and D (LAO cranial views). Coronary angiography (CAG) reveals stenosis in the proximal LCx (white arrowhead, A) and total occlusion of the distal LAD (black arrowhead, A). The right coronary angiogram reveals subtotal occlusion of the posterolateral branch of the RCA (white arrowhead, C). After stent implantation into the LCx, and ballooning of the posterolateral branch, good distal flow was apparent in the absence of residual stenosis (B, D). RAO, right anterior oblique; LAO, left anterio oblique; LCx, left circumflex artery; LAD, left anterior descending artery; RCA, right coronary artery.

Figure 2.
Follow-up coronary angiographic images. A and B (RAO cranial views), and C and D (RAO caudal views). Eight months after the initial intervention, follow-up CAG reveals diffuse stenosis of the distal LAD (black arrowhead, A), and segmental stenosis (up to 95%) in the first diagonal branch of the RCA (white arrowhead, A) and the distal LCx (white arrowhead, C). After successful balloon dilatation, CAG revealed good distal flow without residual stenosis (B, D). RAO, right anterior oblique; CAG, coronary angiography; RCA, right coronary artery; LCx, left circumflex artery.
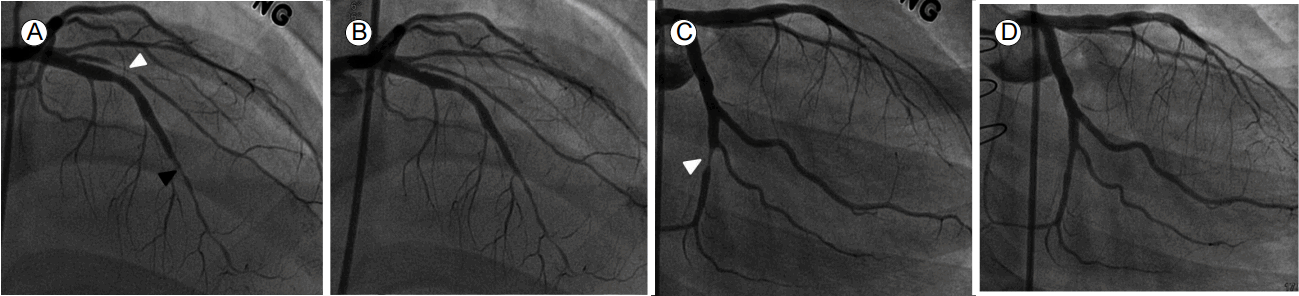
Figure 3.
A gross specimen of the heart (A) and (B-D) the microscopic appearance of the coronary arteries (Masson's trichrome stain). (A) Transverse section of the gross heart. Microscopic examination of the posterior descending branch of the RCA (B, √ó50), the distal branch of the LAD (C, √ó50), and the LCx (D, √ó20) revealed concentric intimal proliferation of smooth muscle cells and less-differentiated spindle cells, indicative of allograft vasculopathy. LAD, left anterior descending artery; RV, right ventricle, LV, left ventricle, PD, posterior descending artery; RCA, right coronary artery; LCx, left circumflex artery.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print




