이온음료 과다 복용으로 촉발된 부신선종 유발 저칼륨혈성 횡문근융해증
Consumption of an Excessive Amount of Ionic Beverage Can Trigger Adrenal Adenoma - Induced Severe Hypokalemic Rhabdomyolysis
Article information
Trans Abstract
Rhabdomyolysis results from acute damage to skeletal muscles caused by various conditions, of which hypokalemia is a recognized but rare example. Although primary aldosteronism may cause severe hypokalemia leading to rhabdomyolysis, the potassium level of such patients can be within the normal range. Hypokalemia is most frequently triggered when these patients are exposed to an additional insult, such as diuretic stress. Similarly, overzealous consumption of ionic beverages with osmotic diuretic effects can cause hypokalemia. Here, we describe a patient with an aldosterone-secreting adrenal adenoma, who presented with acute rhabdomyolysis secondary to severe hypokalemia triggered by consumption of a large volume of ionic beverage for 3 weeks.
INTRODUCTION
Hypokalemia is a recognized but relatively rare cause of rhabdomyolysis. Few reports have described rhabdomyolysis secondary to primary aldosteronism (PA) [1,2]. Although PA may cause severe hypokalemia leading to rhabdomyolysis, patients may have normal potassium levels on routine biochemistry. Hypokalemia can develop due to osmotic diuretic stress caused by consumption of an excessive quantity of ionic beverages for diet control. We experienced a patient with an aldosterone-secreting adrenal adenoma, who presented with acute rhabdomyolysis secondary to severe hypokalemia triggered by consuming a large volume of ionic beverages over 3 weeks.
CASE REPORT
A 42-year-old female presented with acute-onset lower limb paralysis associated with severe proximal muscle pain. She had no medical history of hypertension, drug use or chronic alcoholism but was hypertensive (140-150/90-100 mmHg) at admission. Three weeks prior to admission, she had consumed > 3 L of an ionic beverage daily (carbohydrate, 60 g/L; Na+, 21 mEq/L; potassium (K+), 5 mEq/L; Ca2+, 1 mEq/L; Mg2+, 0.5 mEq/L; Cl-, 16.5 mEq/L; citrate-, 10 mEq/L; lactate-, 1 mEq/L; osmolality, 443 mOsm/L), together with coffee and fruit, to lose weight. A physical examination indicated acute-onset myopathy with symmetric flaccid paralysis of the lower limbs (muscle power, 3/5), with normal muscle power (5/5) in the upper limbs, followed by several days of polyuria. However, no physical findings of dehydration-such as decreased skin turgor, dry tongue or orthostatic hypotension-were discovered at admission.
The initial work-up revealed severe hypokalemia (K+, 1.5 mmol/L) with an elevated creatine kinase (CK) of 17,440 IU (normal, 16-190). Urine was positive for myoglobin with a high urinary myoglobin of 1,037 g/L. Serum creatinine was 0.7 mg/dL. In addition, urinary potassium was elevated (18.5 mmol/L; trans- tubular potassium gradient, 12.5) with metabolic alkalosis (pH, 7.61; HCO3-, 37.2 mmol/L; base excess, 14.7 mmol/L; PCO2, 37 mmHg) at admission. A 24 h urine collection was not performed, and random urine potassium levels were measured on 3 consecutive days (18.5 ≥ 20.2 ≥ 19.7 mmol/L). Other biochemical values at the initial evaluation were serum aspartate aminotransferase, 304 IU/L (normal ≤ 35 IU/L); alanine aminotransferase, 108 IU/L (normal ≤ 38 IU/L); Na+, 144 mmol/L; blood urea nitrogen, 7.4 mg/dL; creatinine, 0.7 mg/dL (normal, 0.6-1.0 mg/dL); Ca2+, 7.4 mg/dL (normal, 8.3-10.3 mg/dL); phosphorus, 1.9 mg/dL (normal, 2.7-4.5 mg/dL); Mg2+, 1.9 mg/dL (normal, 1.6-2.6 mg/dL); fasting blood glucose, 90 mg/dL; and total protein, 7.5 g/dL with albumin of 4.3 g/dL. Thyroid function tests were normal with a free T4 level of 1.0 ng/dL and a thyroid-stimulating hormone level of 0.5 IU/mL.
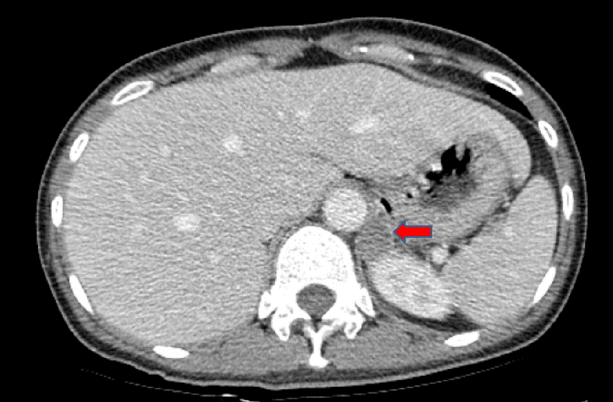
The diagnosis was hypokalemic paralysis and rhabdomyolysis. As soon as the initial laboratory findings of hypokalemia with high urinary potassium loss in association with metabolic alkalosis and high blood pressure were found, we measured plasma renin and aldosterone levels in a supine position. The aldosterone level was 19.96 ng/dL (normal, 1.3-14.5 ng/dL), and the plasma renin activity (PRA) value was below the detection limit of our method (< 0.2 ng/mL/h). The aldosterone/renin ratio (ARR) was 199.6 (normal < 40). An abdominal computed tomography (CT) scan showed a 21 × 19 mm hypodense mass in the left suprarenal gland (Fig. 1). We performed a saline-loading test to obtain a definitive diagnosis of PA. Despite 2 L of normal saline loading for 90 min, the plasma aldosterone level and PRA were 49.82 and < 0.10, respectively, confirming PA. Treatment was initiated with oral and intravenous potassium supplementation. Rhabdomyolysis was treated with isotonic sodium bicarbonate (154 mL/L NaHCO3 in 5% dextrose) and isotonic saline hydration to achieve a urinary pH > 6.5 and hourly urine volume of 300 mL/h. After 10 days, muscular strength and CK levels had returned to normal. Conduction studies and electromyography, which were performed after correcting the hypokalemia, were normal. She underwent left-sided adrenalectomy, and the pathological examination of the gland confirmed a 21 mm adrenal adenoma. The patient was normokalemic without potassium replacement postoperatively, and the PRA was 0.52 ng/mL/h; aldosterone was 1.3 ng/dL with a reduced ARR of 2.5 (< 20 ng/mL/h). Blood pressure was normal 4 weeks after discharge, with no antihypertensive medication, and she was normokalemic with normal muscle strength.
DISCUSSION
It is estimated that PA affects 5-13% of patients with hypertension [3]. Adrenal adenoma is the most common cause of PA, in which there is autonomous and inappropriately high aldosterone production by the adrenal glands, which is not suppressed by sodium loading [4]. Inappropriate production of aldosterone causes hypertension and suppressed plasma renin with sodium retention and potassium excretion, which may lead to severe hypokalemia if prolonged. It is also important to note that only 9-37% of patients with PA reportedly have hypokalemia [5].
This case illustrates several important issues regarding PA and hypokalemia. Although PA leads to hypokalemia due to tubular loss of potassium, in most instances the potassium level may be well within the normal range on a routine biochemical evaluation, as many factors contribute to renal potassium handling. Severe hypokalemia is triggered most frequently when these patients are exposed to an additional insult, such as diuretics [6]. Post-exercise ingestion of glucose-containing sports drinks affects the serum potassium level, resulting in hypokalemia [7]. In addition, a recent report described that drinking large volumes of cola should be added to the differential diagnosis for severe hypokalemia [8], as severe hypokalemia can result from several pathophysiological mechanisms. These perturbations include osmotic diarrhea, osmotic diuresis and hyperinsulinemia secondary to hyperglycemia, with a resulting intracellular to cellular shift in potassium. These effects are magnified by concomitant high levels of caffeine [8]. Cola has 105 g/L carbohydrate. Our patient had consumed a large volume of high carbohydrate (60 g/L), high osmolarity (443 mOsm/L) ionic beverage to lose weight. In our opinion, this beverage has sufficient carbohydrate and osmolality to induce osmotic diuresis, which probably triggered the hypokalemia, leading to rhabdomyolysis. In addition, our patient had hypokalemia with high urinary loss of potassium in association with metabolic alkalosis and high blood pressure, suggesting the possibility of PA. We diagnosed aldosterone-secreting adrenal adenoma based on a saline loading test, abdominal CT scan and postoperative pathological examination.
Here, we described a patient with an aldosterone-secreting adrenal adenoma, who presented with acute rhabdomyolysis secondary to severe hypokalemia triggered by over-consumption of an ionic beverage. This is the first reported case of adrenal adenoma-induced severe hypokalemic rhabdomyolysis caused by an ionic beverage. Therefore, clinicians should note that drinking large volumes of an ionic beverage to lose weight could be harmful and cause osmotic diuresis and hypokalemia.