 |
 |
| Korean J Med > Volume 90(1); 2016 > Article |
|
Abstract
A 58 year-old woman was diagnosed with lung adenocarcinoma (cT3N1M0). We detected a point mutation in epidermal growth factor receptor (EGFR) exon 21 (L858R) and an echinoderm microtubule-associated protein-like 4- anaplastic lymphoma kinase (ALK) rearrangement. The patient was treated with preoperative neoadjuvant chemotherapy and underwent a left lower lobectomy with mediastinal lymph node dissection. However, we could not detect any mutation in EGFR or the ALK rearrangement from the tumor tissue removed. Then, 70 days after completion of adjuvant chemotherapy, she visited our outpatient clinic with diminished visual accuracy and tinnitus. A single brain metastatic lesion was seen on brain magnetic resonance imaging. She underwent surgical removal of the brain mass, which showed a mutation of EGFR, exon 21, but no ALK rearrangement. We report this unusual case of lung adenocarcinoma with a coexisting EGFR mutation and ALK rearrangement, and identify gene alterations before chemotherapy, after chemotherapy, and at recurrence.
Lung cancer remains a leading cause of cancer death worldwide. To date, mutation of the epidermal growth factor receptor (EGFR) gene and rearrangement of the anaplastic lymphoma kinase (ALK) gene are major oncogenic driver mutations, which are key in choosing first-line treatments for patients with lung adenocarcinoma.
EGFR mutations and the ALK rearrangement are found at frequencies of 10-35% and 37% in lung adenocarcinomas, respectively [1]. Although these two genetic alterations had been considered to be mutually exclusive, recent studies have provided evidence that the ALK rearrangement can occur concomitantly with EGFR mutations [2]. Indeed, because patients harboring both EGFR and ALK alterations have been increasingly reported, they have received attention in terms of molecular oncogenesis and therapeutic approaches.
To date, many clinical studies of lung adenocarcinoma patients have determined that EGFR mutations and the ALK rearrangement are the most useful predictive biomarkers of EGFR tyrosine kinase inhibitors (TKI) and ALK inhibitors, respectively. However, it is still unclear whether targeted agents, administered for second-line treatment on the basis of mutation status obtained at the initial diagnosis, are as effective as those for first-line treatment. For patients harboring EGFR mutations, the response rate to EGFR TKIs is lower in second-line than in first-line treatment. The reason for the inconsistency in the predictive value of genetic alterations in EGFR TKIs between first- and second-line treatments remains unknown; however, some researchers have proposed that the influence of first-line chemotherapy on genetic status may be a possible explanation [3].
Recent studies have revealed high intra-tumoral heterogeneity of multiple tumor-suppressor genes and inter-tumoral heterogeneity between primary and corresponding metastatic tumors [4]. Because tumor heterogeneity has a great impact on future anti-cancer treatment strategies, its clinical implications require further investigation.
In this case, we examined the status of EGFR mutations and the ALK rearrangement before chemotherapy, after chemotherapy, and at recurrence, and identified that the genetic alterations at each time point were not identical.
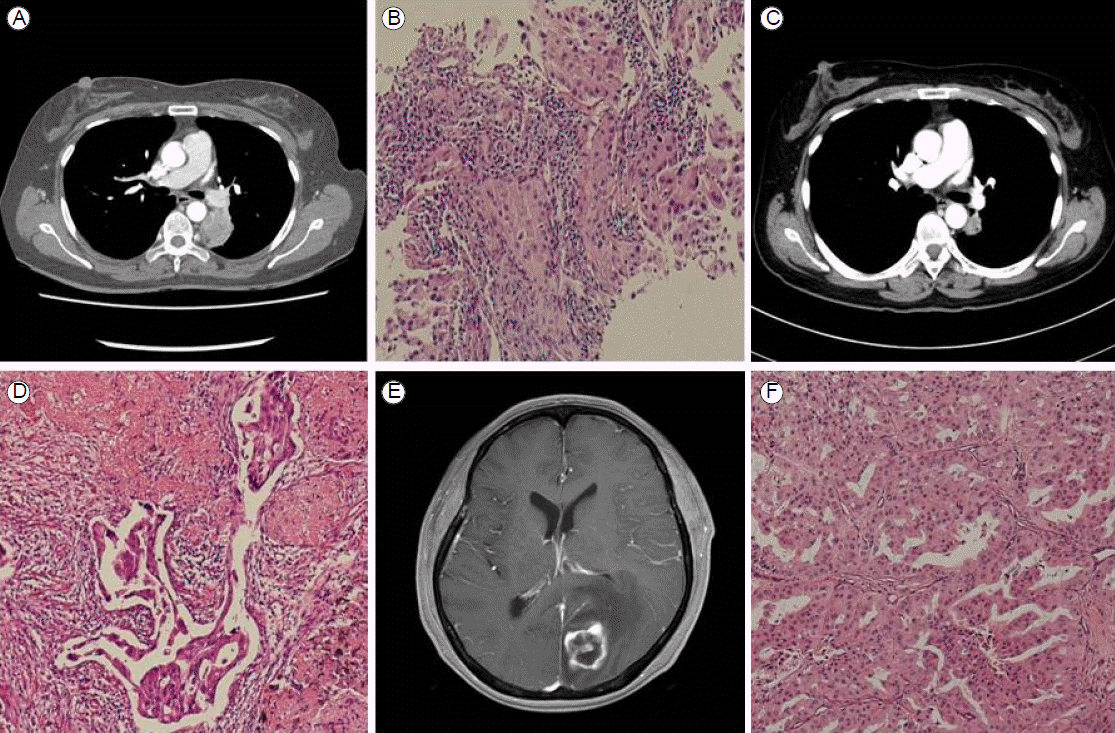
A 58 year-old woman with no smoking history visited our outpatient clinic with a 4-month history of long-lasting coughs in March 2013. A physical examination revealed no abnormality. Laboratory test results were within normal ranges. The serum carcinoembryonic antigen level was 2.49 ng/mL (normal range, 0-4.3 ng/mL). A chest X-ray showed a mass in the left suprahilar area. Computed tomography (CT) of the chest revealed a 39-mm tumor abutting the mediastinal pleura in the superior segment of the left lower lung and metastatic lymph nodes in the left peribronchial region (cT3N1M0; Fig. 1A). We conducted a percutaneous core needle biopsy targeting the left suprahilar mass, and the result of pathological examination was compatible with a primary lung adenocarcinoma (Fig. 1B).
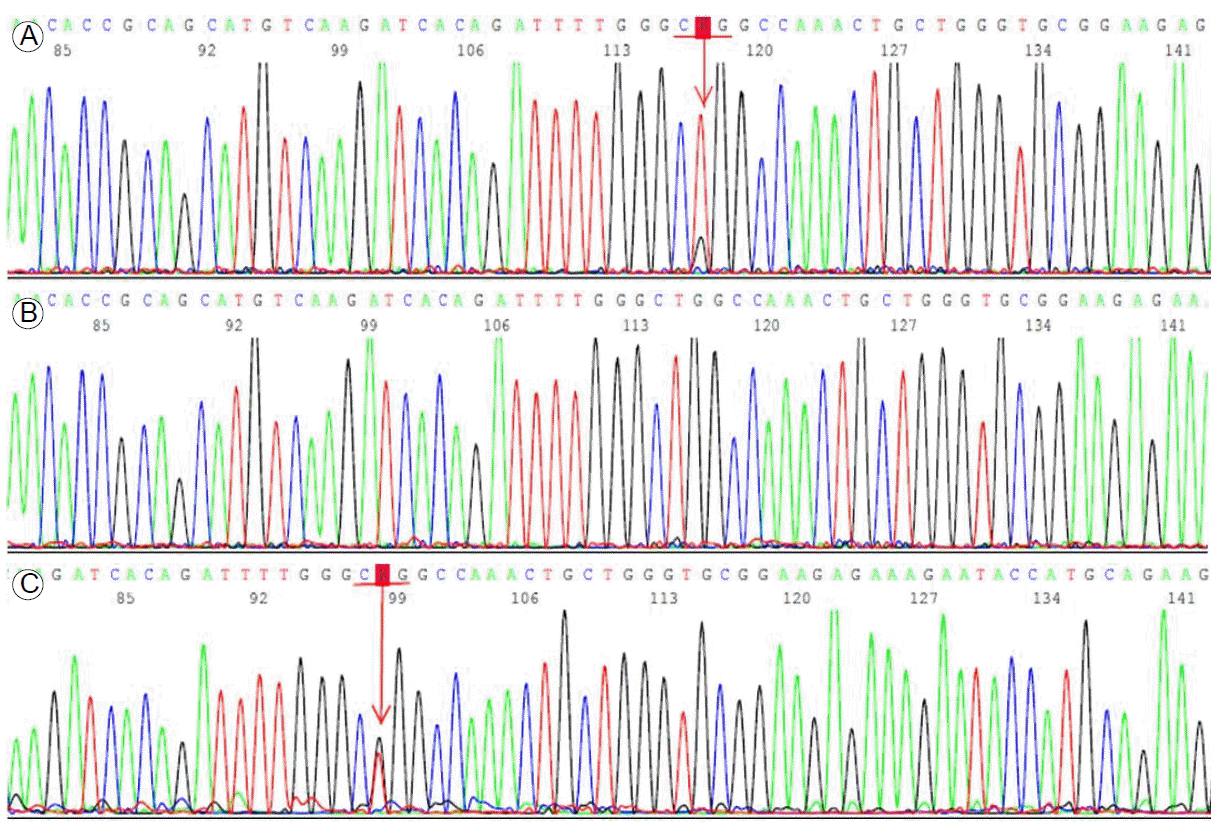
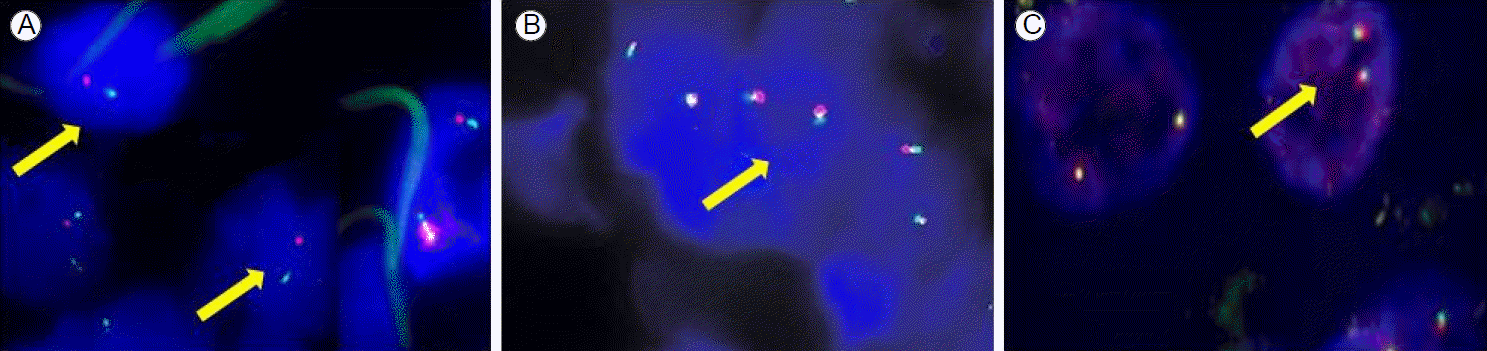
We analyzed the status of EGFR/K-ras mutations and the ALK rearrangement in paraffin wax-embedded tumor tissue by direct sequencing. Genomic DNA extracted from the tumor sample was used for Sanger sequencing of EGFR exons 18-21. These exons were amplified by polymerase chain reaction (PCR), and the PCR products were purified and labeled for sequencing using the BigDye 3.1 kit (Biosystems, Barcelona, Spain) according to the manufacturerтАЩs protocol. The ALK rearrangement was also examined by fluorescent in situ hybridization (FISH) using LSI ALK Dual Color, Break Apart Rearrangement Probes (Vysis, IL, USA). ALK FISH is generally accepted as positive when more than 15% of 50 analyzed cells have split off fluorescent probes flanking the ALK locus. We detected a point mutation in EGFR exon 21 (L858R) as well as the echinoderm microtubule-associated protein-like 4-ALK rearrangement with a frequency of 20% in the tumor tissue (Figs. 2A, 3A). However, there was no K-ras mutation.
The patient was treated with preoperative neoadjuvant chemotherapy, including cisplatin (80 mg/m2) and docetaxel (60 mg/m2), every 3 weeks, up to two cycles. She tolerated the treatment well, and follow-up chest CT and fludeoxyglucose positron emission tomography scans demonstrated partial radiological and metabolic responses in the primary lung mass (a decrease from 4.0 ├Ч 3.0 ├Ч 4.2 to 2.5 ├Ч 1.4 ├Ч 2.5 cm) with a complete radiological and metabolic response of the metastatic lymph nodes in the left peribronchial area (Fig. 1C). After that, she underwent a left lower lobectomy with mediastinal lymph node dissection. The status of EGFR mutations and the ALK rearrangement were also examined in the surgically removed tumor tissue. However, we did not detect any mutations in EGFR exons 18-21 or the ALK rearrangement (Figs. 2B, 3B). According to the pathological staging (pT1aN1Mx; Fig. 1D), the patient received adjuvant chemotherapy, including cisplatin (80 mg/m2) and docetaxel (60 mg/m2) every 3 weeks, up to three cycles.
Then, 70 days after completion of adjuvant chemotherapy, she visited our outpatient clinic with several daysтАЩ history of diminished visual acuity in the right eye and tinnitus. A single brain metastatic lesion was seen on brain magnetic resonance imaging shortly thereafter (Fig. 1E). She was transferred to the Department of Neurosurgery and underwent surgical removal of the brain mass. The pathological diagnosis of the removed tumor was a metastatic adenocarcinoma from the primary lung cancer (Fig. 1F). We examined the EGFR mutation and ALK rearrangement in the metastatic tumor tissue, which revealed a point mutation of EGFR exon 21 (L858R) (Fig. 2C), but no ALK rearrangement (Fig. 3C).
Over the last decade, significant advances have been made in the identification of oncogenic driver mutations playing key roles in tumor initiation and progression in non small cell lung carcinoma (NSCLC). Among the well-known driver mutations, EGFR mutations and ALK fusions have been regarded as biological determinants for the selection of specific targeted agents [5,6]. Generally, these two genetic alterations have been considered to be mutually exclusive [7]. However, the coexistence of these two oncogenic driver mutations has been reported in rare cases (0.330.97%) [8]. The proportion of patients harboring concomitant EGFR and ALK alterations is currently increasing, resulting from clinical application of highly sensitive detection tools for EGFR mutations [9].
This case was a female patient with a lung adenocarcinoma harboring both an EGFR mutation and an ALK rearrangement at the initial presentation. It was notable that the genetic alterations were different in the primary tumor before chemotherapy, after surgical removal, and at recurrence (Table 1). The molecular study of this case supports the theory that chemotherapy may have an effect on gene alterations in tumor tissue. Both the EGFR mutation and ALK rearrangement in the primary tumor changed to the wild type after neoadjuvant chemotherapy, indicating that the number of cancer cells harboring these genetic alterations may have been decreased significantly by induction chemotherapy. Based on this, it is suggested that although the tumor cell populations harboring EGFR mutations and ALK rearrangements responded well to cytotoxic chemotherapy, the cell population without gene alterations still remained, which were replaced by wild-type tumor cells derived from genetic alteration-positive tumor cells. Our observations were similar to those of a previous study [3]. These results support the theory of intra-tumoral heterogeneity. Moreover, only if the cells carrying EGFR mutations to the total tumor cells constitute more than 10-20% would the result of direct sequencing be positive. If malignant cells remained in very small areas, the result might be a false negative. Not to miss the existence of an EGFR mutation or ALK rearrangement would require careful microdissection of each slide section. However, the examination of each section in the entire tumor tissue is unlikely in clinical practice.
Genetic alterations after chemotherapy can provide important clues to determining the optimal sequence of anticancer treatment: targeted agents followed by cytotoxic chemotherapy versus cytotoxic chemotherapy followed by targeted agents for metastatic lung adenocarcinomas harboring major oncogenic driver mutations. In our case, first-line cytotoxic chemotherapy induced genetic alterations, including an EGFR mutation and an ALK rearrangement. If targeted agents were chosen as first-line drugs, they might have yielded the highest antitumor efficacy for a patient harboring genetic alterations. Further investigations are needed to confirm our results.
In our case, the EGFR mutation in the primary tumor was consistent with that of the corresponding metastatic tumor in the brain, whereas the ALK rearrangement was not. Although molecular mechanisms underlying metastatic progression have not yet been clearly determined, it has been suggested that EGFR mutation-positive clones with enhanced metastatic potential and resistance to chemotherapy may give rise to brain metastases. It is still unclear whether administration of EGFR TKIs, instead of cytotoxic chemotherapy in the adjuvant setting, can effectively reduce recurrence risk in NSCLC patients harboring EGFR mutations.
It remains controversial whether EGFR mutations and ALK rearrangements coexist in the form of single or multiple clones. In our case, EGFR mutations alone were found in the metastatic brain tumor, indicating that multiple clones may be more likely. It is conceivable that adjuvant EGFR TKIs after curative surgery may suppress potential micrometastatic clones harboring EGFR mutations in lung adenocarcinomas. Novel diagnostic methods are required to detect potential metastatic clones in peripheral blood for the selection of appropriate targeted drugs in the adjuvant setting and for the prediction of recurrence earlier during the follow-up period.
As shown in our case and a previous study [10], single-gene alterations in primary tumors are not always the same in recurrent metastatic lesions. These results indicate that assessment of EGFR mutations and the ALK rearrangement in specimens collected at the initial diagnosis may be inappropriate for predicting tumor responses to targeted agents for second-line treatment. Because targeted agents are being used widely to treat recurrent or metastatic disease, more aggressive tissue sampling from recurrent or metastatic sites is recommended to accurately determine gene alterations.
In summary, we experienced a rare case of lung adenocarcinoma with coexistence of an EGFR mutation and an ALK rearrangement, and identified gene alterations before chemotherapy, after chemotherapy, and at recurrence. This case may help our understanding of the molecular mechanisms for different genetic alterations induced by cytotoxic chemotherapy in primary and corresponding metastatic tumors.
REFERENCES
1. Jang TW, Oak CH, Chang HK, Suo SJ, Jung MH. EGFR and KRAS mutations in patients with adenocarcinoma of the lung. Korean J Intern Med 2009;24:48тАУ54.



2. Tiseo M, Gelsomino F, Boggiani D, et al. EGFR and EML4-ALK gene mutations in NSCLC: a case report of erlotinib-resistant patient with both concomitant mutations. Lung Cancer 2011;71:241тАУ243.


3. Bai H, Wang Z, Chen K, et al. Influence of chemotherapy on EGFR mutation status among patients with non-small-cell lung cancer. J Clin Oncol 2012;30:3077тАУ3083.


4. Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012;366:883тАУ892.

5. Ji H, Li D, Chen L, et al. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell 2006;9:485тАУ495.


6. Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693тАУ1703.

7. Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247тАУ4253.



8. Lee JK, Kim TM, Koh Y, et al. Differential sensitivities to tyrosine kinase inhibitors in NSCLC harboring EGFR mutation and ALK translocation. Lung Cancer 2012;77:460тАУ463.


(A) Initial chest CT. A 39-mm tumor abutting the mediastinal pleura in the superior segment of the left lower lung (cT3N1M0). (B) The biopsy specimen at low magnification (├Ч100). (C) Chest CT after neoadjuvant chemotherapy. (D) Pathological examination of the lobectomy specimen. Malignant cells and atrophy are seen at low magnification (├Ч100). (E) Brain MRI of the same patient after treatment. A rim-enhancing nodule is seen in the left occipital lobe. (F) Brain specimen. Malignant cells and atrophy are seen at low magnification (├Ч100). CT, computed tomography; MRI, magnetic resonance imaging.
Figure┬а1.
Representative electropherograms of the EGFR gene. (A) EGFR exon 21 Leu858Arg (c.2573T>G) is seen in the primary tumor specimen (arrow). (B) No Leu858Arg mutation is observed in the exon 21 of the EGFR gene obtained from the lung tissue after neoadjuvant chemotherapy. (C) EGFR exon 21 Leu858Arg (c.2573T>G) is seen in the brain metastasis (arrow). EGFR, epidermal growth factor receptor.
Figure┬а2.
Fluorescent in situ hybridization (FISH). (A) A split of two probes (arrows) that flank the ALK translocation site in EML4-ALK-positive tissue. (B) Fusion of two signals (arrow), representing normal copies of the ALK gene in the lung tissue after chemotherapy. (C) Fusion of two signals (arrow), representing normal copies of the ALK gene in the brain metastasis. ALK, anaplastic lymphoma kinase; EML4, echinoderm microtubule-associated protein-like 4.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print




