INTRODUCTION
Tumor necrosis factor-alpha (TNF-╬▒) is a proinflammatory cytokine that plays an important role in the pathogenesis of inflammatory diseases, such as ankylosing spondylitis (AS). Infliximab, an anti-TNF monoclonal antibody, suppresses inflammation by blocking TNF-╬▒ activity, resulting in dramatic improvements in the signs and symptoms of AS [1]. However, prolonged use of anti-TNF therapy has been associated with numerous adverse effects, including serious infections and lymphoma, of which tuberculosis infections are most problematic [2]. Here, we report a rare case of tuberculous peritonitis in a patient with AS on infliximab therapy without evidence of latent infection before initiating infliximab therapy.
CASE REPORT
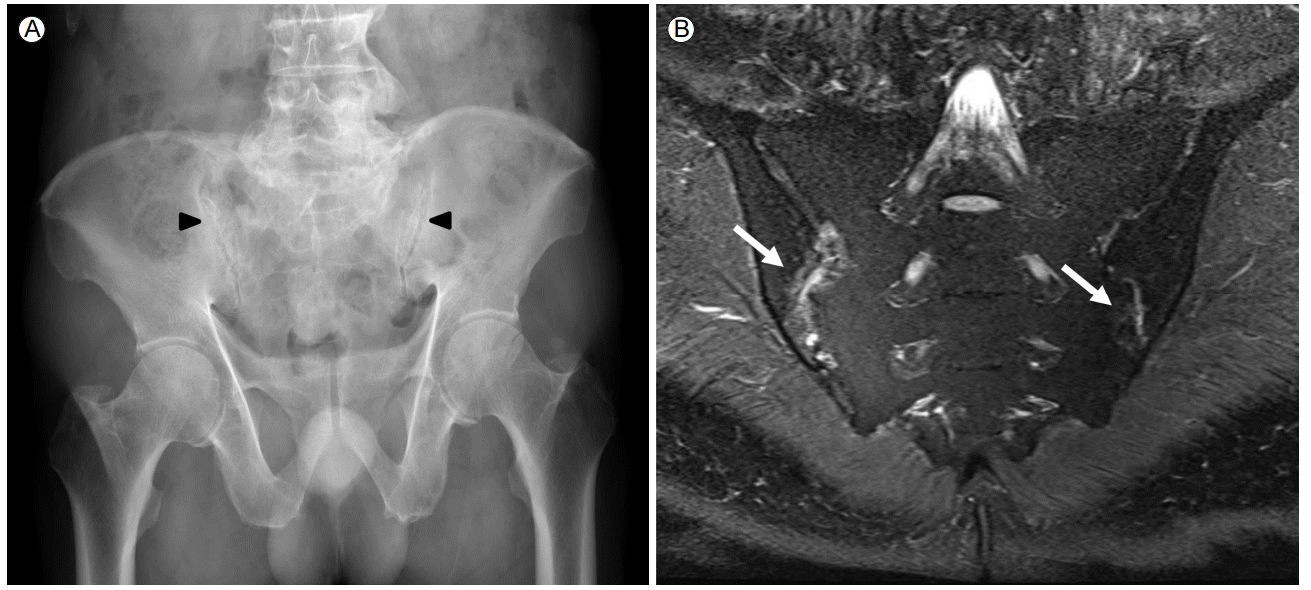
A 71-year-old male with AS was admitted to the hospital with abdominal distension and fever, which had persisted for one week. He was initially diagnosed with AS in March 2013, following complaints of left ankle pain with swelling along with inflammatory back pain. At the time of diagnosis, a pelvic plain X-ray (Fig. 1A) and magnetic resonance imaging (Fig. 1B) revealed active sacroiliitis. Erythrocyte sedimentation rate (ESR) was 120 mm/h (normal, 0-15 mm/h) and C-reactive protein (CRP) was 10.71 mg/dL (normal, 0.01-0.47 mg/dL); HLA-B27 was positive. He was initially treated with celecoxib, sulfasalazine, and methotrexate for three months. Because the treatment response was incomplete, infliximab treatment was began in June 2013. Screening for tuberculosis was performed before initiation of infliximab therapy, using a combination of chest x-ray, tuberculin skin test (TST), and interferon-╬│ release assay (IGRA). The chest x-ray was normal, and the TST and IGRA were negative. Consequently, infliximab therapy was started without tuberculosis prophylaxis. Infliximab (300 mg) was administered intravenously 11 times bi-monthly over the next 18 months. Infliximab therapy was effective, and the AS disease activity had diminished significantly prior to the most recent admission.
At the time of admission, the patientŌĆÖs blood pressure was 113/70 mmHg, heart rate was 100 beats/min, and body temperature was 37.9┬░C. His weight was 58 kg, which was unchanged in recent months, and his height was 167 cm. No specific past medical, family, or social histories were disclosed. On physical examination, chest auscultation revealed normal breathing and heart sounds. The abdomen was soft and distended. Laboratory evaluations showed a white blood cell count of 5,950/mm3 (normal, 4,000-10,000/mm3) with 63.1% neutrophils (normal, 50-75%), and a hemoglobin level of 13.4 g/dL (normal, 13.0-18.0 g/dL). ESR was 31 mm/h and CRP was 17.95 mg/dL. Electrolytes, hepatic function tests, and renal function tests were all within normal limits. Hepatitis virus and human immunodeficiency virus serology were negative. No organisms were detected in blood cultures.
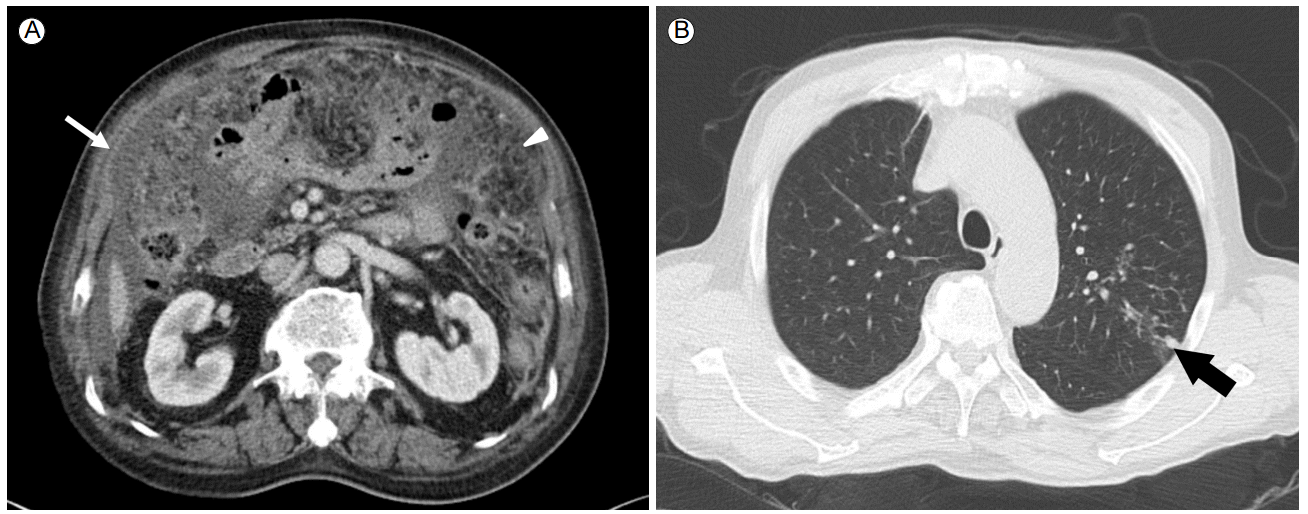
An abdominal computed tomography (CT) scan revealed massive ascites, peritoneal thickening, and a smudged appearance of the omentum (Fig. 2A). The ascites contained 2,560/mm3 white blood cells with 40% lymphocytes, and elevated adenosine deaminase (ADA) at 97.4 IU/L (normal, < 40 IU/L). Ascites cultures for bacteria and Mycobacterium tuberculosis and cytology results were negative. Sputum cultures were also negative for M. tuberculosis.
A laparoscopic biopsy was performed to confirm the diagnosis. Multiple nodules were detected throughout the peritoneum and omentum, which were analyzed using multiple biopsies. Histopathological analyses revealed chronic granulomatous inflammation with caseous necrosis and multinucleated giant cells (Fig. 3). Polymerase chain reaction (PCR) analysis for M. tuberculosis was positive in the biopsy specimens.
Although the chest x-ray revealed no active lung lesions, we performed chest CT to identify the site of primary infection, which revealed a small infiltrative lesion in the left upper lobe (Fig. 2B). Bronchoscopy showed no endobronchial lesions. No acid-fast bacilli were seen, and PCR of the sputum for M. tuberculosis was negative, while PCR of the bronchoalveolar lavage fluid was positive.
Infliximab was stopped and anti-tuberculosis therapy (isoniazid 300 mg/day, rifampicin 600 mg/day, ethambutol 1,200 mg/day, and pyrazinamide 1,500 mg/day) with methylprednisolone (1 mg/kg) was initiated. Following initiation of therapy, the abdominal distension with ascites decreased and the symptoms improved.
DISCUSSION
Infliximab is an intravenously administered monoclonal antibody used to treat several chronic inflammatory diseases, including AS, via direct inhibition of TNF-╬▒. Although TNF-╬▒ inhibition has been shown to dramatically improve clinical outcomes in patients with AS, increased susceptibility to infectious diseases, particularly tuberculosis, remains a major concern, and patients require careful monitoring throughout infliximab therapy [2]. Because TNF-╬▒ plays a key role in granuloma formation in the pathogenesis of tuberculosis, inhibition of TNF-╬▒ may enable tuberculosis progression. Although a number of different TNF blockers have been developed, the risk of tuberculosis infection differs between drug classes. Monoclonal antibodies, such as infliximab and adalimumab, are associated with a higher incidence of tuberculosis compared with soluble TNF receptors, such as etanercept. This observation is likely due to differences in the structure and mechanism of action of each drug class [3].
Compared with other autoimmune diseases, AS is typically associated with a younger age of onset (< 40 years); however, initial diagnosis in older patients (> 50 years), as seen in this case, is also observed, classified as late-onset AS (LOAS). Compared with early-onset AS, LOAS patients are more likely to suffer from combined peripheral arthritis and inflammatory systemic symptoms, and have high ESR and CRP levels, though they lack the typical axial symptoms commonly seen in younger patients. Management of LOAS is often complicated due to the rarity of the condition and the advanced age of the patients [4]. Treatment options for LOAS are typically the same as those used for patients with early onset AS (NSAIDs, sulfasalazine, and TNF-╬▒ blockers). However, the effects of aging on drug metabolism and pharmacokinetics, in combination with other comorbidities and polypharmacy, cause difficulties in the therapeutic management of LOAS patients [5]. Careful surveillance is required when using immunosuppressive drugs in this age group, particularly with regards to infectious diseases, such as tuberculosis.
Tuberculous peritonitis is an uncommon manifestation of tuberculosis. The mechanism of infection is thought to be the hematogenous spread of M. tuberculosis from active pulmonary lesions, ingestion of contaminated sputum or food, or the activation of a latent tuberculosis infection within the peritoneum. Chen et al. [6] showed that development of active tuberculosis is characterized by continuously rising levels of interferon-╬│ or IGRA conversion in rheumatoid arthritis (RA) patients receiving a TNF-╬▒ blocker. In that study, latent tuberculosis infection (LTBI) reactivation is thought to have occurred during the first 3 months after starting TNF-╬▒ blocker therapy. Considering the long interval between initiation of TNF-╬▒ blocker therapy and onset of active tuberculosis, a recent exposure to tuberculosis infection is often the most probable cause of active tuberculous disease for immunocompromised patients living in an area with a high or intermediate tuberculosis burden [6]. In the case described here, tuberculous peritonitis was diagnosed 18 months after beginning infliximab therapy. Although we did not perform a follow-up IGRA, the timing of this infection suggests a primary tuberculosis infection rather than reactivation of latent tuberculosis, indicating a hematogenous spread or ingestion of M. tuberculosis as the cause. High-risk factors for progression to active disease after infection include immunosuppressive therapy, certain medical disorders (e.g., acquired immunodeficiency syndrome (AIDS), diabetes, severe kidney disease, malignancy, and malnutrition), and old age [7]. Our patient was elderly and on immunosuppressive therapy, consistent with a high risk of active tuberculosis infection.
The symptoms of tuberculous peritonitis, including abdominal pain and distension, anorexia, fever, and weight loss, are nonspecific, with conclusive diagnoses generally possible only after M. tuberculosis is suspected. Only three cases of tuberculous peritonitis have been reported in patients treated with infliximab in Korea; two had RA and the other had AS [8-10]. In these cases, tuberculous peritonitis developed within six months after initiation of infliximab, with only one case confirmed by peritoneal biopsy. In the other two cases, diagnosis of tuberculosis peritonitis was based on radiological findings and ascites ADA levels, without an adequate peritoneal biopsy. Unlike these prior cases, this case of tuberculous peritonitis was not diagnosed until 18 months after initiation of infliximab. Given the age and condition of our patient, we were unable to exclude malignancies such as peritoneal carcinomatosis, thereby requiring a biopsy to make a definitive diagnosis.
In patients at high risk for tuberculosis being treated with a TNF-╬▒ blocker, strict surveillance for tuberculosis infection with serial chest radiographs is warranted, in combination with the lowest dose of immunosuppressive drugs possible to achieve a therapeutic response. Because tuberculosis can present in a variety of ways, with or without pulmonary involvement, both a high degree of suspicion and a diagnostic biopsy are required to make a definitive diagnosis of extrapulmonary tuberculosis. Here, we reported a patient with AS presenting with a rare form of tuberculosis. The rarity of this infection highlights the need to always consider the possibility of tuberculosis in AS patients being treated with infliximab.
Infliximab is a chimeric monoclonal antibody used to treat several chronic inflammatory diseases, including AS, via direct inhibition of TNF-╬▒. However, the use of TNF blockers comes with several serious side effects, including a decreased ability to fight infections, such as tuberculosis. In the case presented here, tuberculous peritonitis is thought to have occurred as a result of hematogenous spread of M. tuberculosis from active pulmonary lesions, or through ingestion of contaminated sputum. Given both the rarity of tuberculous peritonitis and the difficulty in diagnosing this condition, this unusual case serves to remind physicians to consider tuberculosis in patients who present with ascites during infliximab therapy.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print






