한국 남성 노인에서 발견된 췌장 고형 가유두상 종양의 증례
A First Case of Solid Pseudopapillary Tumor of the Pancreas in an Old Man in South Korea
Article information
Trans Abstract
Solid pseudopapillary tumor (SPT) of the pancreas is a rare tumor that typically affects young women without causing significant clinical symptoms. No case of SPT in an old man has been reported in South Korea, and such cases are very rare worldwide. We report a 70-year-old man with SPT of the pancreas with multiple organ metastasis. Although surgical resection is the treatment of choice for SPT, we decided not to treat, considering his age and the disease severity.
INTRODUCTION
A solid pseudopapillary tumor (SPT) is an epithelial tumor of the pancreas with low malignant potential that predominantly occurs in young women [1]. The American pathologist Virginia Frantz first described it in 1959, and this neoplasm was officially named “solid pseudopapillary tumor” by the World Health Organization (WHO) in 1996 [2].
The histogenesis of SPT remains unclear, but acinar, endocrine, ductal, and progenitor cells have all been suggested as possible origins of this tumor [3]. SPT is usually a mixture of solid, cystic, hemorrhagic, and necrotic components morphologically [4].
Ultrasonography (US), X-rays, computed tomography (CT), and magnetic resonance imaging (MRI) can be used as diagnostic tools [1], and surgical resection is the treatment of choice [5].
In old men, SPT is often associated with diagnostic and therapeutic challenges because this tumor is typically observed in young women. We report the case of a 70-year-old man with an SPT and review the literature.
CASE REPORT
A 70-year-old man was admitted to Seoul Veterans Hospital with a 3-month history of dyspnea and weight loss (5 kg). Routine laboratory tests were all within the normal range: white blood cell count, 9,410/μM; hemoglobin, 12.5 g/dL; platelets, 350,000/μL; Na, 136 mEq/L; K, 4.8 mEq/L; and Cl, 98 mEq/L. The serum neuron-specific enolase (NSE) level was elevated at 38 ng/mL. On physical examination, there was mild diffuse abdominal tenderness.
The chest X-ray showed several nodules in the left lower lobe (LLL) that were overlooked initially because they overlapped the heart and diaphragm edge (Fig. 1). Chest CT also showed several nodules in the LLL along the left diaphragmatic pleura, which were thought to be metastasis with multiple metastatic lymphadenopathy in the anterior diaphragmatic area (Fig. 2).

Chest computed tomography showed several nodules in the left lower lobe along the left diaphragmatic pleura.
Abdomen and pelvis CT showed a 16 × 10 cm heterogenic multilobulated soft/cystic tissue mass in the left upper abdomen invading the pancreatic tail, spleen, second portion of the duodenum, gallbladder, and hepatic flexure of the colon, with calcification and necrotic changes. There were also multiple metastatic hypervascular low-attenuation lesions in the liver that showed progressive enhancement with gradual “fill-in enhancement” in the venous phase (Fig. 3).

Abdomen and pelvis computed tomography showed a pancreatic tail mass and a huge aggregated mass with necrotic portions and some bulging contours in the liver and spleen.
The diagnosis was confirmed by a transhepatic US-guided gun biopsy, which was consistent with secondary involvement of SPT from a pancreas primary. Immunohistochemistry was positive for CD10, CD56, cyclin D1, and PR, focally positive for pan CK, and negative for CK7, CK20, TTF-1, and chromogranin A (Fig. 4).
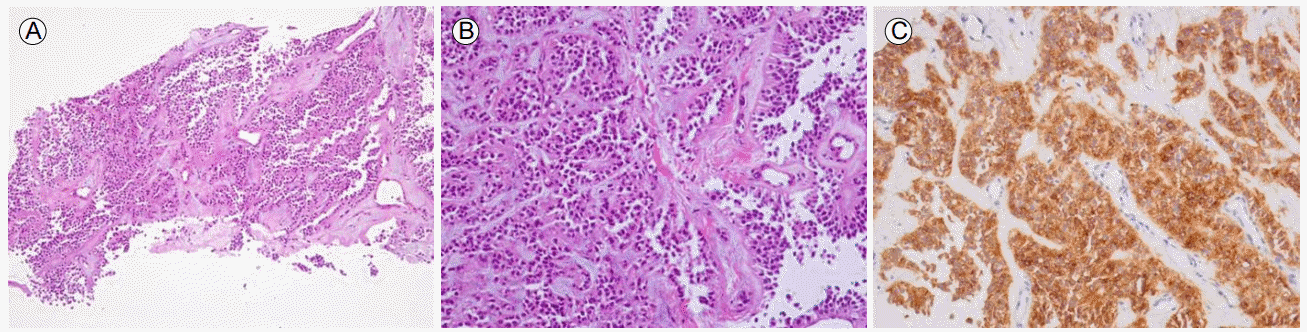
Pathological features of solid pseudopapillary tumor. (A) The tumor showed the typical pseudopapillary pattern (×100). (B) Solid areas contained a monotonous cell population with myxohyaline stroma (×200). (C) Immunohistochemistry revealed strong CD10 expression (×200).
Considering his age and disease severity, we decided not to operate with the patient’s consent. He has been followed as an outpatient for 7 months, although follow-up CT shows that the tumor has grown.
DISCUSSION
Solid pseudopapillary tumor has also been called solid pseudopapillary neoplasm, papillary cystic tumor, and solid papillary epithelial neoplasm, before being defined as “solid pseudopapillary tumor” of the pancreas by the WHO in 1996 [2,6]. It is a very unusual tumor that constitutes 5% of the pancreatic neoplasms, and 0.2-2.7% of the primary non-endocrine tumors, of the pancreas [7,8].
Unlike pancreatic cancer, SPT is observed most frequently in young women, with the greatest incidence in the second and third decades (female: male ratio, 20:1) [9].
Most patients present with vague symptoms related to tumor compression of the adjacent organs, including abdominal discomfort, mild abdominal pain, increased abdominal girth, poor appetite, and nausea; almost 30% of patients with SPT are asymptomatic [6,10]. SPT tends to be misdiagnosed because of the non-specific symptoms, especially in elderly men.
The chest images showed only minimal nodules, and arterial blood gas analysis showed no evidence of hypoxemia. Retrospectively, the patient might have expressed his abdominal discomfort as dyspnea. However, the metastatic nodules could have provided a diagnostic clue to a major problem in his abdomen, if we had looked closely at the blind spot around the heart and diaphragm.
On CT, SPT typically appears as a large, heterogeneous, mixed solid/cystic, encapsulated lesion with visible peripheral calcifications and progressive enhancement, with gradual intralesional “fill-in enhancement” in the portal and venous phases after contrast administration. The high resolution of MRI will show the presence of the capsule and intratumoral hemorrhage better than multidetector CT [5].
A fine-needle aspiration (FNA) biopsy is a definitive diagnostic tool, but should be done carefully due to the danger of peritoneal seeding and the risk of complications, such as bleeding or pancreatic and biliary fistulas [5].
Histologically, SPT has solid areas that alternate with a pseudopapillary pattern and cystic spaces, which are the results of gradual degenerative changes occurring in the solid neoplasm [6].
The immunohistochemical pattern of SPT is distinctive. Positive SPT markers include vimentin, alpha-1-antitrypsin, CD10, and CD56. SPT may also reveal focal immunoreactivity for cytokeratin, NSE, synaptophysin, and progesterone receptors [6].
Despite its low-grade malignant potential and the favorable prognosis of the disease, an estimated 15% of adult and 13% of pediatric SPT cases are malignant [5]. Most metastases are found in the liver at the time of diagnosis, with an incidence of 15%, and local recurrence is rare in the long-term follow-up of these patients [8]. SPT tends to proliferate locally, but it can also infiltrate surrounding structures (70% of cases), especially the vena cava, splenic vein, spleen, diaphragm, stomach, omentum, and retroperitoneum, as in our case [9].
Surgical resection is the ultimate treatment of choice. Distal pancreatic resection is performed if the tumor is located in the body or tail of the pancreas, while a pancreatoduodenectomy using the Whipple or Traverso–Longmire procedure is performed if the tumor is located in the head of the pancreas [1]. The presence of metastases in SPT is not a negative prognostic factor and these lesions should be removed surgically [1,9]. The 1-, 3-, and 5-year survival rates following radical surgery are 99.4%, 97.5%, and 96.5%, respectively [3].
Chemo- or radiotherapy is another treatment option. Several experimental regimes have been used without a significant clinical response, including 5-fluorouracil, doxorubicin, streptozotocin, cisplatin, topotecan, ifosfamide, and etoposide. A favorable response to radiotherapy in locally advanced unresectable disease has been reported [10].
Solid pseudopapillary tumor is not a common disease, especially in older men who complain of atypical symptoms. SPT in an old man has not been reported in Korea. Metastatic lung lesions should suggest that clinicians check abdominal/pelvic CT, and the final diagnosis can be confirmed with FNA biopsy. Fortunately, the prognosis of SPT is favorable compared with other common cancers, although a delayed diagnosis necessitates aggressive treatment.
The prognosis of SPT in an old man may be worse than in a young woman because of a missed or delayed diagnosis. A high index of suspicion must be kept to enable an early diagnosis of SPT and treatment.
