 |
 |
| Korean J Med > Volume 89(3); 2015 > Article |
|
Abstract
Only two cases of gastric intramural hematoma (IMH) caused by endoscopic mucosal resection (EMR) have been reported to date. This is the first reported case of gastric IMH caused by EMR, treatment of which required hemoclipping and transcatheter arterial embolization. The patient had a normal coagulation profile and no relevant medical history. About 8 h after completing the EMR, the patient vomited approximately 150 mL fresh blood and complained of abdominal pain. Endoscopy showed a 3 Г— 7 cm hematoma with active surface bleeding in the gastric antrum. Hemoclipping of the bleeding site on the surface and transcatheter arterial embolization of the left gastric artery were performed. Thereafter, conservative management including administration of a proton pump inhibitor was performed, and the lesion resolved. A review of relevant previous cases and this case suggested vessel damage secondary to the submucosal injection itself to be a reasonable causative mechanism for the gastric IMH.
Intramural hematoma (IMH) of the gastrointestinal tract can result from anticoagulant therapy, coagulopathy, trauma, pancreatic disease, or unknown causes [1]. Gastric IMH is less common than esophageal or duodenal IMH, and that caused by endoscopic mucosal resection (EMR) is very rare [1,2]. Only two cases of gastric IMH caused by EMR have been reported to date. This is the first reported case of gastric IMH that required hemoclipping and transcatheter arterial embolization because of active bleeding on the surface of the hematoma and unstable vital signs [3,4].
A 54-year-old female underwent upper gastrointestinal endoscopy to screen for gastric cancer at a secondary referral center, and a polyp of the gastric antrum was noted. Pathologic examination of a tissue sample obtained from the lesion through endoscopic forceps biopsy was neither specific nor diagnostic as the pathologic appearance of the tissue suggested chronic active gastritis with foveolar epithelial hyperplasia. The patient was recommended to undergo resection of the lesion for both diagnosis and treatment, for which she visited our hospital.
The patient had no significant medical or surgical history. Laboratory evaluations performed before the procedure showed normal results (hemoglobin level, 13.8 g/dL; platelet count, 198 K/ВөL; prothrombin time, 10.4 s [104%, 0.97 international normalized ratio]; activated partial prothrombin time, 34.9 s). On the morning of the procedure for resection of the lesion, 40 mg pantoprazole was administered intravenously to reduce the risk of bleeding during and after the procedure. The polyp, located on the greater curvature of the antrum, was approximately 0.7 Г— 0.7 cm in size, ovoid in shape, and subpedunculated (Fig. 1A).
The polyp was lifted by injecting approximately 4 mL solution containing 9.5 mL normal saline, 0.2 mL epinephrine diluted 1:1,000, and 0.3 mL indigo carmine under the polyp (Fig. 1B) using an injection needle (Wilson instruments Co., Ltd., Shanghai, China). The base of the lesion, specifically where the polyp was in contact with the surface of the inner wall of the stomach, was grasped with a snare (Finemedix Co., Ltd., Daegu, Korea) and resected using 1-2 s electric current flow (in endocut Q mode; cut duration, 1; cut interval, 4; and effect 2 with forced coagulation mode of effect 1) through the snare using an electrosurgical unit (VIO300D, ERBE, TГјbingen, Germany). After the resection, a white coagulated wound was observed. No immediate bleeding occurred (Fig. 1C). However, the operator placed three hemoclips (Olympus Co., Ltd., Tokyo, Japan) to reduce the risk of delayed postoperative bleeding. The hemoclipping almost completely closed the bed formed by the resection (Fig. 1D). A second 40 mg dose of intravenous pantoprazole was administered 6 hr after the procedure to reduce the risk of procedure-related bleeding.
About 8 hr after completion of the procedure, the patient vomited about 150 mL fresh blood and complained of epigastric pain. Her heart rate increased from 70 to 110 beats/min, and her systolic/diastolic blood pressure increased from the normal range to 150/90 mmHg. Emergency endoscopy showed retention of a large amount of fresh blood and a 3 Г— 7 cm club-like hematoma with active bleeding on its surface on the great curvature of the antrum (Fig. 2A and 2B). A wound at the site of hemoclipping performed after the EMR was found near the base of the hematoma (Fig. 2C). The operator placed 10 hemoclips at the bleeding site on the surface of the hematoma to slow the bleeding, which eventually stopped (Fig. 2D). A further angiography was performed because the bleeding was presumed to be continuing inside the hematoma, although it appeared to have stopped on the surface. On the angiogram, active extravasation of contrast medium was not evident (Fig. 3A), but prophylactic embolization of the left gastric artery was performed (Fig. 3B).
Then, conservative management including parenteral-only nutrition, blood transfusion, and intravenous administration of a proton pump inhibitor (pantoprazole) was commenced. Endoscopy 3 days after the procedure showed a large amount of blood, a blood clot, and slight oozing at the site of the previous hemoclipping on the surface of the hematoma (Fig. 4A). However, an additional hemostasis procedure was not performed because the patientвҖҷs condition, including her vital signs, was stable, and the examiner believed that the hematoma contained not blood, but a blood clot, based on its springy but solid texture, dark green color, and crumpled, not tense appearance (Fig. 4B). Conservative management was continued, and an oral diet was commenced 6 days after the procedure. An endoscopy performed 10 days after the procedure showed that the lesion had disappeared, and the layer of the area corresponding to the base of the hematoma had peeled away. The lesion was undergoing the healing process with inflammation (Fig. 4C and 4D). The post-EMR pathologic report indicated that the resected tissue was not neoplastic, but merely a fibrous lump.
Gastric IMH caused by endoscopic procedures is rare. One endoscopic procedure that reportedly causes gastric IMH is injection therapy for hemostasis of bleeding peptic ulcers. The other responsible procedures are those associated with EMR [5,6]. Only four cases of EMR-associated gastric IMH have been reported to date (Table 1). Two of the four cases were of gastric IMH that developed after EMR [3,4]. In the third case, IMH was caused by only the submucosal injection itself, which was performed to lift the lesion prior to resection with electric current; in the fourth case, IMH developed after submucosal injection and argon plasma coagulation (APC) for additional therapy for possible residual tumor tissue, following a previous EMR for gastric adenoma [7,8].
The authors of some previous case reports have suggested that gastric IMH develops as the blood leaking from arterial vessels damaged by electric current or by APC fills the space produced by submucosal injection prior to resection of the lesion [3,8]. However, vessel damage caused by the submucosal injection itself seems to be a more likely mechanism than vessel damage caused by electric current or APC. This is because (i) the gastric IMH developed immediately after the submucosal injection and before the electric current was provided at the resection site in one of the four EMR-associated cases, (ii) submucosal injection was performed in all four reported cases of EMR-associated gastric IMH, and (iii) cases of gastric IMH development due to only injection therapy for hemostasis in bleeding peptic ulcers without electrocautery have been reported [5-7]. A common theme among the three previously reported cases of gastric IMH after EMR or APC [3,4,8] and the present case is that neither the resection sites nor the APC wounds were around the center of the hematomas; instead, all were located at the periphery of, or adjacent to, the hematomas (Fig. 2C). This further supports the notion that vessel damage caused by the submucosal injection itself, rather than the effect of electrocautery, is the most likely causative mechanism of gastric IMH. A wound caused by bleeding from vessels damaged by electrocautery is more likely to form around the center of the hematoma.
With conservative management, including parenteral-only nutrition and administration of a proton pump inhibitor, the lesions resolved without adverse events in three of the four above-mentioned cases of EMR- or APC-associated gastric IMH that did not have active bleeding on the surface of the hematoma [3,7,8]. A surgical operation was needed in only one case with active surface bleeding [4]. However, hemoclipping for active bleeding on the surface of the IMH and transcatheter arterial embolization was required in the present case because of continuous active bleeding and unstable vital signs (increased blood pressure and heart rate). In summary, conservative management may suffice for a case of EMR- or APC-associated gastric IMH without active bleeding on the surface of the hematoma, while vascular intervention or surgery may be required for a case with active surface bleeding. The angiogram in the present case did not show active extravasation of the contrast medium. This may be attributable to the previous hemoclipping performed on the surface of the IMH to slow the bleeding. Nonetheless, the operator performed embolization of the left gastric artery because the bleeding was expected to be continuing inside the hematoma although it seemed to have stopped on the surface. If a transcatheter arterial embolization is not performed, bleeding might recur on the surface due to increased intrahematoma pressure caused by continuous bleeding inside the hematoma. Previous reports support the validity of prophylactic transcatheter arterial embolization in upper gastrointestinal bleeding without extravasation seen on an angiogram. Lang et al. [9] compared the results of groups that did and did not undergo prophylactic embolization among patients with massive upper gastrointestinal hemorrhage and normal findings on angiography. They demonstrated that lesions not treated with embolization or other invasive therapies had a high rate of massive recurrent hemorrhage, and concluded that prophylactic embolization of the left gastric artery is warranted when there is definite prior identification of a lesion in the left gastric artery territory or there is no prior localization of a lesion but the patient is at risk of multiorgan failure if bleeding recurs. Padia et al. [10] confirmed the effects of prophylactic transcatheter arterial embolization in upper gastrointestinal bleeding without extravasation seen on an angiogram; however, a high proportion of the included cases had recently undergone surgery and underwent the procedure on the gastroduodenal artery, unlike the present case who had no surgical history and involvement of the left gastric artery. They compared the results of arterial embolization between two groups with and without contrast medium extravasation among patients with acute upper gastrointestinal hemorrhage and found that the proportion of patients requiring additional blood transfusion or surgery, 30-day hemorrhage-related mortality, and treatment success rates did not differ significantly between the two groups.
EMR-associated gastric IMH is a rare condition. However, operators should be aware of the possibility of this adverse event during endoscopic procedures, and should closely evaluate resected tissue and apply preventive procedures, such as electrocoagulation of denuded vessels, if necessary, because EMR is currently performed frequently worldwide and gastric IMH may be fatal.
REFERENCES
1. Hughes CE 3rd, Conn J Jr, Sherman JO. Intramural hematoma of the gastrointestinal tract. Am J Surg 1977;133:276вҖ“279.


2. Dhawan V, Mohamed A, Fedorak RN. Gastric intramural hematoma: a case report and literature review. Can J Gastroenterol 2009;23:19вҖ“22.



3. Lee CK, Jang JY, Hwangbo Y, et al. A case of gastric huge intramucosal hematoma after snare polypectomy. Korean J Gastrointest Endosc 2008;36:219вҖ“223.
4. Sun P, Tan SY, Liao GH. Gastric intramural hematoma accompanied by severe epigastric pain and hematemesis after endoscopic mucosal resection. World J Gastroenterol 2012;18:7127вҖ“7130.



5. Sadio A, Peixoto P, Cancela E, et al. Intramural hematoma: a rare complication of endoscopic injection therapy for bleeding peptic ulcers. Endoscopy 2011;43 Suppl 2 UCTN:E141вҖ“E142.

6. Rohrer B, Schreiner J, Lehnert P, Waldner H, Heldwein W. Gastrointestinal intramural hematoma, a complication of endoscopic injection methods for bleeding peptic ulcers: a case series. Endoscopy 1994;26:617вҖ“621.


7. Yang CW, Yen HH. Large gastric intramural hematoma: unusual complication of endoscopic submucosal dissection. Endoscopy 2011;43 Suppl 2 UCTN:E240.

8. Keum B, Chun HJ, Seo YS, et al. Gastric intramural hematoma caused by argon plasma coagulation: treated with endoscopic incision and drainage. Gastrointest Endosc 2012;75:918вҖ“919.


(A) An approximately 0.7 Г— 0.7 cm subpedunculated polyp was seen on the greater curvature of the gastric antrum. (B) The polyp was lifted by injecting about 4 mL solution of normal saline, diluted epinephrine, and indigo carmine under the polyp. (C) After resection, a white, coagulated wound was observed. (D) Hemoclipping was performed three times, almost completely closing the bed formed by the resection.
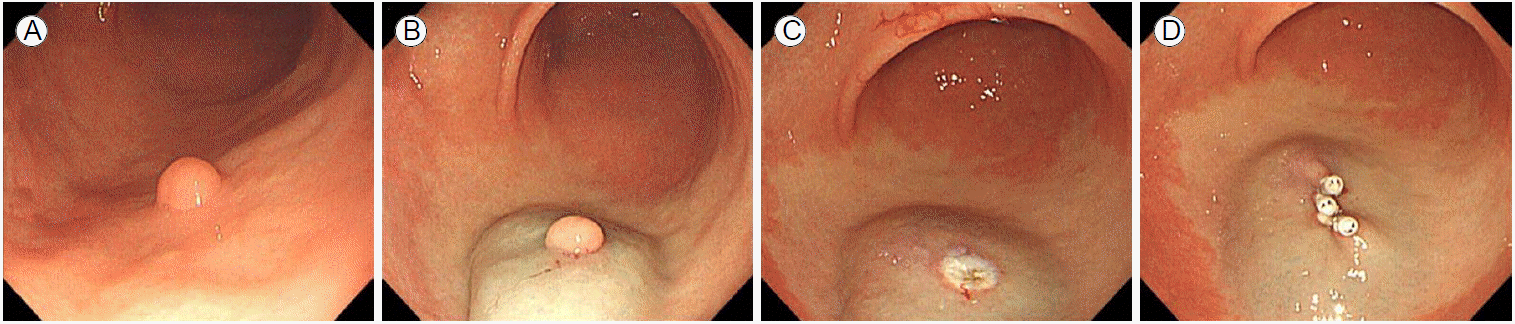
FigureВ 1.
(A, B) Emergency endoscopy showed retention of a large amount of fresh blood and a 3 Г— 7 cm club-like hematoma with active bleeding on its surface. (C) The wound at the site of hemoclipping performed after resection of the polyp (indicated with arrow) was found near the base of the hematoma. (D) Hemoclipping was performed at the site of bleeding on the surface of the hematoma.

FigureВ 2.
(A) The arrows indicate the left gastric artery. (B) The arrows indicate the disappearance of blood flow through the left gastric artery after transcatheter arterial embolization.

FigureВ 3.
(A) Endoscopy performed 3 days after the procedure showed a large amount of blood, a blood clot, and slight oozing at the site of a previous hemoclipping on the surface of the hematoma. (B) The hematoma was not springy, but solid; dark green in color; crumpled, and not tense on endoscopy 3 days after the procedure. (C, D) Endoscopy performed 10 days after the procedure showed that the lesion had disappeared, the layer of the area corresponding to the base of the hematoma had peeled away, and the lesion was undergoing the healing process; inflammation was also evident.
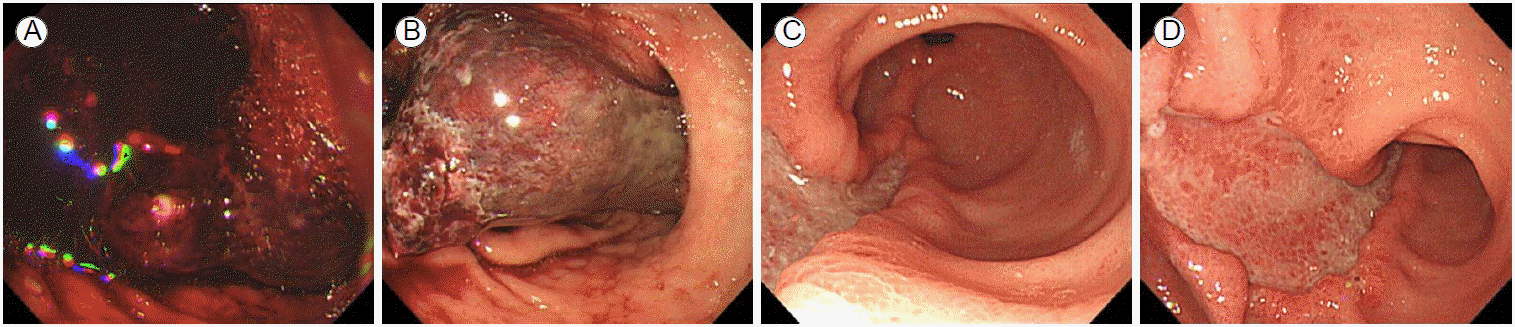
FigureВ 4.
TableВ 1.
Cases of gastric intramural hematoma associated with endoscopic mucosal resection, argon plasma coagulation, or submucosal injection
| First author | Sex/age | Morphology of the lesion | Location of the lesion | Procedure for resection | Pathologic diagnosis | Treatment method |
|---|---|---|---|---|---|---|
| Lee et al. [3] | Female/58 | 5 mm sessile | Antrum | SI, SP with CF | Hyperplastic polyp | Conservative management |
| Sun et al. [4] | Male/33 | 0.4 Г— 0.5 cm protrusive | Antrum | SI, SP with CF | Tubular adenoma | Surgical operation |
| Yang et al. [7] | Female/72 | Subepithelial | Antrum | Only SI | - | Conservative management |
| Keum et al. [8] | Female/71 | Post-ESD wound | Distal body | SI, APC | - | Incision of the hematoma and suction |
| Park et al. (this report) | Female/54 | 0.7 Г— 0.7 cm polypoid | Antrum | SI, SP with CF | Fibrous lump | Hemoclipping and TAE |
-
METRICS

-
- 1 Crossref
- 0 Scopus
- 8,969 View
- 133 Download



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



