|
 |
| Korean J Med > Volume 79(1); 2010 > Article |
|
Abstract
Myelofibrosis is a myeloproliferative neoplasm characterized by abnormal bone marrow megakaryocyte proliferation with reticulin and collagen fibrosis, leukoerythroblastosis, anemia, increased level of serum lactate dehydrogenase and splenomegaly. Myelofibrosis associated with malignant lymphoma is rare and survival rates appear to have been poor. Herein, we describe our experience in a patient who remained in complete remission with high-dose therapy (HDT) with autologous peripheral blood stem cell transplantation (PBSCT) for ALK-negative ALCL presenting with rapidly progressing myelofibrosis. (Korean J Med 79:77-81, 2010)
Anaplastic lymphoma kinase (ALK)-negative anaplastic large cell lymphoma (ALCL) evidences a poor prognosis similar to that of peripheral T-cell lymphoma, unspecified (PTCL-NOS)1). Myelofibrosis has been previously described as being characterized by fibrosis with excessive deposits of extracellular matrix proteins, and secondary myelofibrosis occurs in a variety of malignant hematopoietic disorders2). T-cell lymphoma with myelofibrosis has previously been described in a limited number of articles3-8). All of these patients were treated with a CHOP (cyclophosphamide, Vincristine, doxorubicin, prednisone)-like regimen, but their survival rates appear to have been poor. None of these patients underwent autologous hematopoietic stem cell transplantation. Herein, we describe our experience in a patient who remained in complete remission with high-dose therapy (HDT) with autologous peripheral blood stem cell transplantation (PBSCT) after the application of conventional chemotherapy for ALK-negative ALCL presenting with rapidly progressing myelofibrosis.
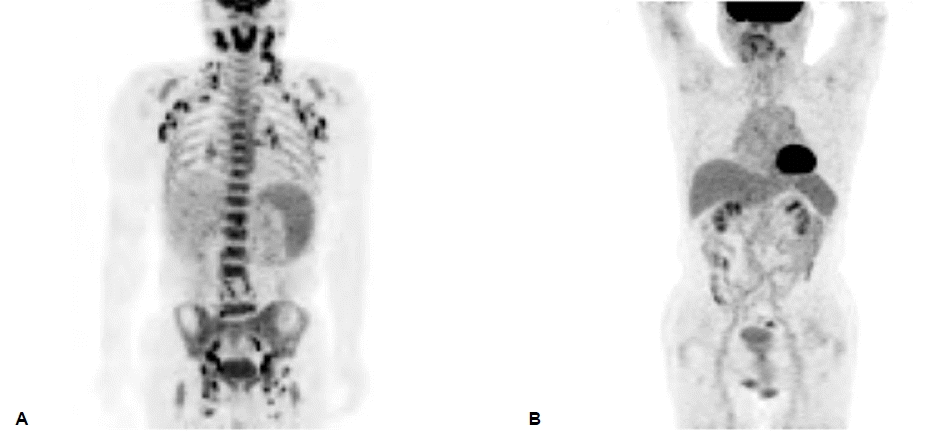
A 55-year-old male was admitted to our hospital for anemia and idiopathic fever. The patient complained of an intermittent fever and fatigue which had persisted for 3 weeks. Upon physical examination, he evidenced pallor and multiple lymph node enlargement of approximately 1 or 2 cm in both the cervical and inguinal areas. The patient’s complete blood count was abnormal, with hemoglobin 7.5 g/dL, a white blood count (WBC) of 4.84×109/l, and 257×109/l platelets. Lactate dehydrogenase (LDH) was increased (555 IU/l, normal 218-472 IU/l). The results of positron emission tomography/computed tomography (PET/CT) indicated strong 18F-fluorodeoxyglucose (18F-FDG) uptake at the nasopharynx, oropharynx, spleen, bone marrow, and multiple lymph node areas in the cervical, axillary, mediastinal, abdominal, retroperitoneal and inguinal sites, bilaterally. Under the suspicion of lymphoma, a lymph node biopsy was conducted on the cervical and inguinal areas, which revealed polymorphous neoplastic lymphoid cells with atypical nuclei (Fig. 1). Immunohistochemical stains of the lymph node biopsy evidenced the following tumor-cell immunophenotypes: CD45RO+, EMA+, CD30+, CD3+, CD15-, CD10-, CD1a-, CD56-, CD20- and ALK-. Bone marrow aspiration was conducted by dry tap. The bone marrow examination revealed marked reticulin fibrosis, dysplastic megakaryocytic hyperplasia, and the suppression of normal hematopoiesis (Fig. 2A-C). Based on these findings, we diagnosed the patient as having ALCL of ALK negative with myelofibrosis. After 2 weeks of hospitalization, cytopenia (WBC 1.08×109/l, hemoglobin 8.0 g/dL, platelets 2×109/l) progressed rapidly, and chemotherapy with CHOP was initiated. His lymphadenopathy and fever rapidly regressed and cytopenia was mildly improved. But, nineteen days after chemotherapy, lymph nodes were enlarged and fever was recurred. Immediately, he was treated with biweekly CHOP plus Etoposide (CHOEP) with the support of granulocyte colony-stimulating factors (G-CSF). Fortunately, following of the third cycle of dose-dense CHOEP,cytopenia was normalized and lymph nodes weren’t palpated. After the fifth cycle of CHOEP, PET/CT evidenced no hypermetabolic lesions and the bone marrow biopsy evidenced an absence of fibrosis with hypocellular hematopoietic tissue of 20~30% in cellularity (Fig. 2D). Autologous stem cells were mobilized with cisplatin, cytarabine, and dexamethasone (DHAP) plus 5 mg/kg/day G-CSF, and 3.29×106 CD34+ cells/kg were collected. On October 23, 2006, the patient underwent autologous PBSCT after conditioning with intravenous busulfan (from days -8 to -5; 3.2mg/kg per day), etoposide (from days -5 to -4; 400 mg/m2 per day) and cyclophosphamide (from days -3 to -2; 60 mg/kg per day). He received G-CSF from day +1 to +20 to accelerated granulocyte recovery. The absolute neutrophil count reached 0.5×109/l and the patient’s platelet count reached 50×109/l on days +20 and +25, respectively. The course of the transplantation was generally uneventful, with good engraftment, and the patient was sent home after four weeks of hospitalization. After 36 months of Autologous PBSCT, the patient remained in complete remission on PET-CT (Fig. 3) and was alive as of October 2009, with no progression of the disease.
Malignant lymphoma with diffuse myelofibrosis is a vanish-ingly rare condition. No cases of ALCL with myelofibrosis have yet been reported. There have been only 6 cases of myelofibrosis associated with peripheral T-cell lymphoma reported3-8). The majority of the patients evidenced pancytopenia, splenomegaly, and increased levels of LDH (Table 1). In chronic idiopathic myelofibrosis, various cytokines, such as platelet-derived endothelial growth factor (PDGF), transforming growth factor-β1 (TGF-β1), basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) play an important role in the development of stromal proliferation2). In T-cell lymphoma with myelofibrosis, previous studies reported elevated serum TGF-β1, PDGF and bFGF4,7,8). However, the pathogenesis of myelofibrosis in non-Hodgkin’s lymphoma is unknown.
All of these patients were treated with intensive chemotherapy, as a result, improvement of myelofibrosis and/or recovery of hematopoiesis was observed in four cases3-6). However, 2 patients was relapsed with lymphoma and myelofibrosis5,6). Only one of 6 patients underwent allogeneic hematopoietic stem cell transplantation and remained alive with no progression of the disease for 16 months4). Currently, no standard therapy for the treatment of these conditions is available. In the present case, the patient was only 55 years old, and had a good performance status. He also evidenced rapidly progressive pancytopenia, splenomegaly and multiple lymph node involvement. We treated him with a regimen of dose-dense CHOEP and his lymph node enlargement and myelofibrosis were improved. With regard to allogeneic stem cell transplantation, the patient had no compatible donor. Fortunately, autologous peripheral blood stem cell mobilization was performed successfully and he underwent autologous PBSCT with HDT. This treatment allowed clinical and hematological remission over the next 36 months. The case reported here suggests that autologous PBSCT with HDT can be an effective treatment in patients with T-cell lymphoma with diffuse myelofibrosis.
Acknowledgements
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (0620220-1).
REFERENCES
1. ten Berge RL, de Bruin PC, Oudejans JJ, Ossenkoppele GJ, van der Valk P, Meijer CJ. ALK-negative anaplastic large-cell lymphoma demonstrates similar poor prognosis to peripheral T-cell lymphoma, unspecified. Histopathology 43:462–469, 2003.


3. Takai K, Sanada M. Peripheral T-cell lymphoma initially presenting as secondary myelofibrosis. Rinsho Ketsueki 30:2199–2204, 1989.

4. Abe Y, Ohshima K, Shiratsuchi M, Honda K, Nishimura J, Nawata H, Muta K. Cytotoxic T-cell lymphoma presenting as secondary myelofibrosis with high levels of PDGF and TGF-beta. Eur J Haematol 66:210–212, 2001.


5. Uehara E, Tasaka T, Matsuhashi Y, Fujita M, Tamura T, Shimoura Y, Mano S, Kuwajima M, Nagai M. Peripheral T-cell lymphoma presenting with rapidly progressing myelofibrosis. Leuk Lymphoma 44:361–363, 2003.


6. Rao SA, Gottesman SR, Nguyen MC, Braverman AS. T cell lymphoma associated with myelofibrosis. Leuk Lymphoma 44:715–718, 2003.


Lymph node biopsy showing polymorphous neoplastic lymphoid cells with atypical nuclei (H&E stain, ×400).

Figure 1.
Pathological features of bone marrow. (A) The bone marrow at initial diagnosis is hypercellular and show increased numbers of megakaryocytes that are atypical (H&E stain, ×400). (B) There are reticulin fibers illustrates the marked reticulin fibrosis (Silver stain, ×200). (C) Small amounts of collagen fibers are laid down in the bone marrow (Masson's trichrome stain, ×200). (D) The bone marrow is hypocellular but trilineage hematopoietic cells are within normal limit in distribution (H&E stain, ×400).
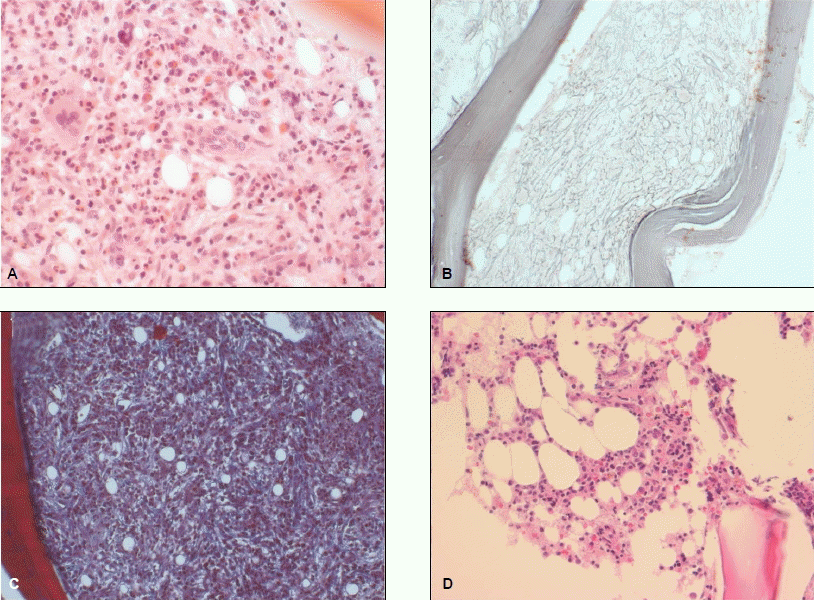
Figure 2.
Table 1.
Hematological and clinical features of T-cell lymphoma with myelofibrosis
WBC, white blood cell count; Hb, hemoglobin; LDH, lactate dehydrogenase; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisolone; THP-COP, pirarubicin hydrochloride, cyclophosphamide, vincristine, prednisolone; Allo, allogeneic peripheral blood stem cell transplantation; Auto, autologous peripheral blood stem cell transplantation; NG, not given.
- TOOLS
-
 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link XML Download
XML Download Full text via DOI
Full text via DOI-
 Download Citation
Download Citation
 Print
Print-
Share :