INTRODUCTION
A reported patient with viral fulminant myocarditis was admitted with seriously impaired cardiac function requiring aggressive hemodynamic support and discharged after several weeks with markedly improved systolic function [1]. After surviving the acute unstable period, the long-term outcome of this disease is excellent [2]. Heart transplantation is occasionally required if the patient does not recover from the acute period; otherwise, activity is limited post-recovery. The optimal recovery time for a successful outcome in patients with fulminant myocarditis is the early period after presentation. We treated a 54-year-old diabetic man presented with fulminant myocarditis and was considered for cardiac transplantation. However, he survived without transplantation and his conduction disturbance and systolic function had improved 6 months after presentation. Late conduction recovery on the electrocardiogram (ECG) with improved systolic function in patients with acute fulminant myocarditis is rare. Here, we report such a case with a literature review.
CASE REPORT
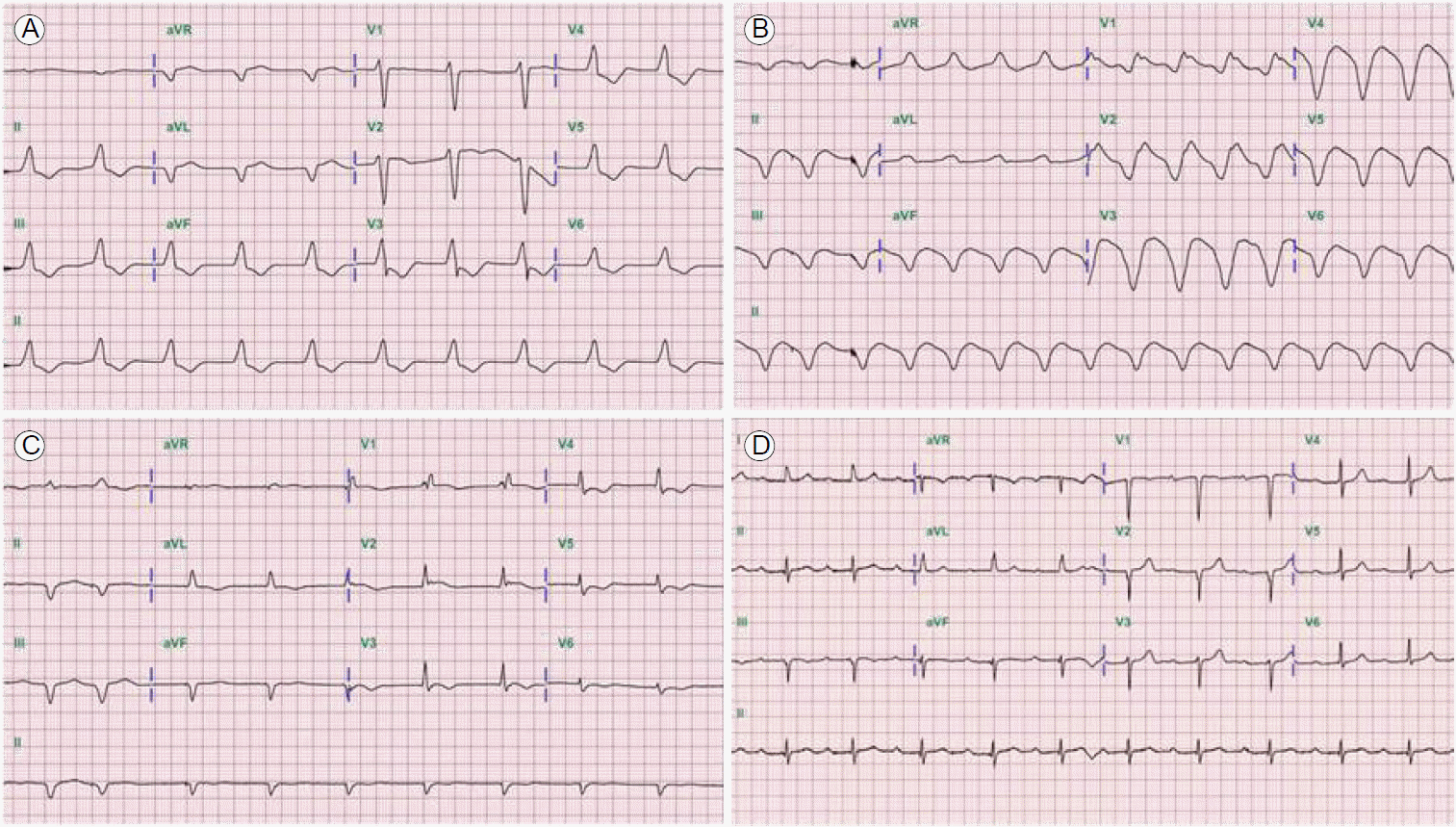
A 54-year-old man whose body mass index was 26.2 kg/m2 presented to our hospital with dyspnea (New York Heart Association class IV). He had a medical history of diabetes and hypertension for 5 years, and he had chills, a cough, and general aches for 1 week before presentation. The physical examination demonstrated unstable vital signs: blood pressure 79/53 mmHg, pulse rate 60 beats per minutes, respiratory rate 28 breaths per minute, and body temperature 37┬░C. An initial ECG showed a junctional escape rhythm with nonspecific intraventricular conduction delay (QRS duration, 166 msec; Fig. 1A and 2A). Chest x-rays indicated congestion of both lung fields, and laboratory tests revealed elevated N-terminal pro-B-type natriuretic peptide (32,724 pg/mL) and cardiac biomarkers including troponin-T (3.98 ng/mL), creatine kinase (CK, 313 U/L), and CK-MB (44.5 ng/mL). Other biochemical tests indicated multi-organ failure (aspartate aminotransferase, 342 U/L; alanine aminotransferase, 890 U/L; blood urea nitrogen, 83 mg/dL; and creatinine, 2.03 mg/dL). An echocardiogram showed severe global hypokinesia of the left ventricle (left ventricular end diastolic dimension, 54 mm; left ventricular ejection fraction [LVEF], 15%, using the Simpson method) with a thickened myocardium. However, the coronary angiography findings were normal.
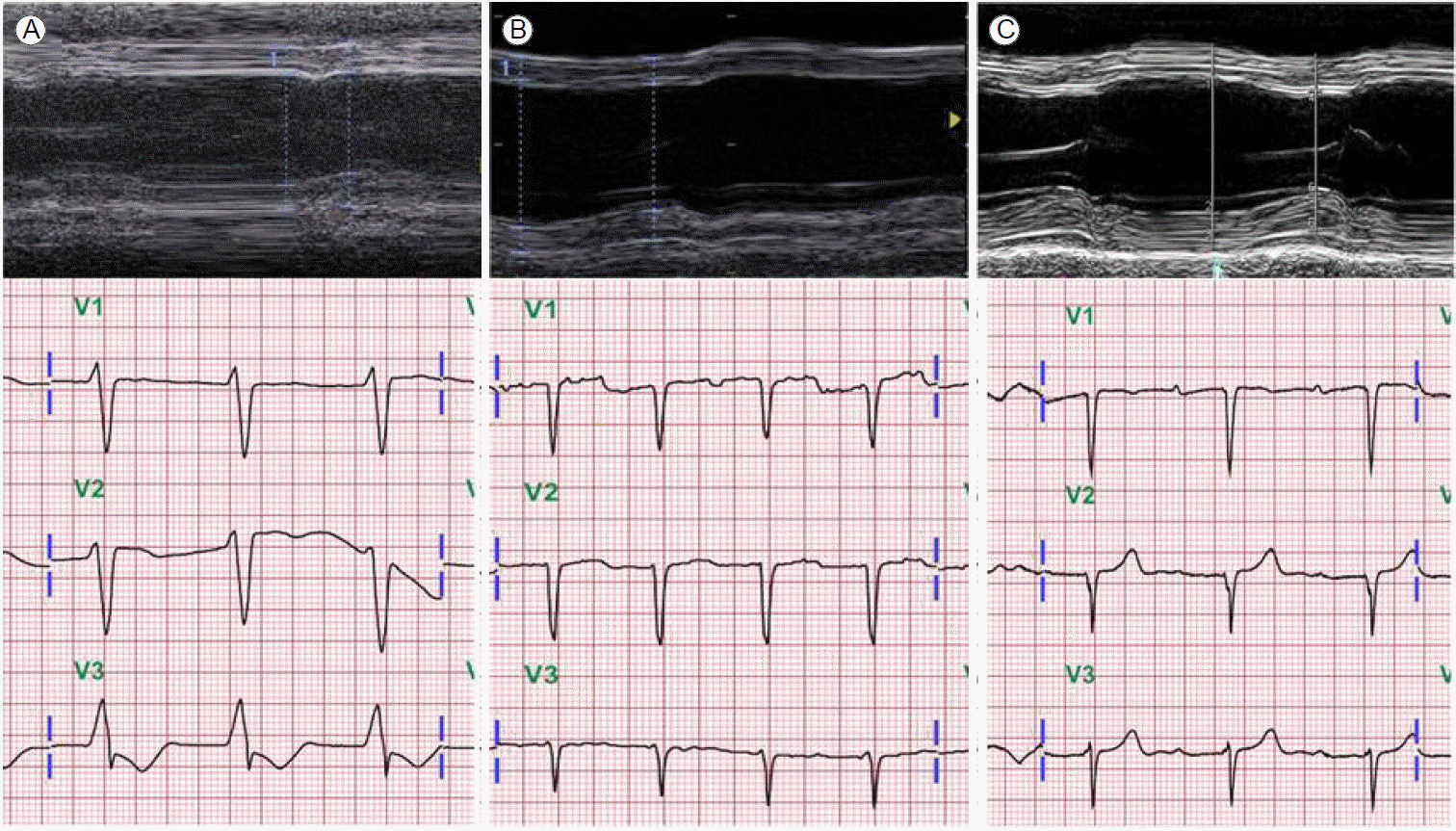
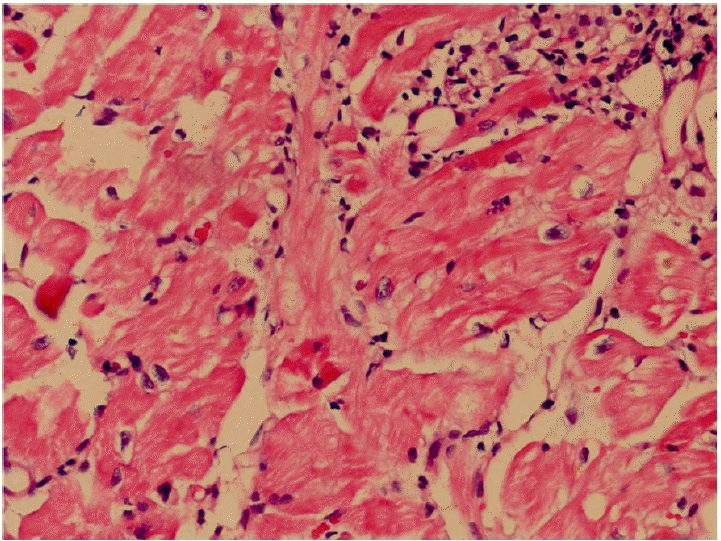
Immediate emergency mechanical circulatory support was initiated using extracorporeal membrane oxygenation (ECMO). On the third day of ECMO support, an ECG showed a markedly widened QRS complex (QRS duration, 254 msec; Fig. 1B), while the peak systole to peak diastole blood pressure was nearly flat (< 5 mmHg). We tried to maintain a mean blood pressure > 65 mmHg with mechanical and medical hemodynamic support. Cardiac magnetic resonance imaging was not conducted due to frequent ventricular tachycardia and fibrillation. An endomyocardial biopsy was performed to confirm the diagnosis; serial endomyocardial biopsies revealed significant myocyte necrosis with myocardial fibrosis in every specimen (Fig. 3). Due to cardiac rhythm instability and intractable blood pressure, we administered immunosuppressive therapy with intravenous methylprednisolone 1,000 mg per day for 3 days. While no clinical improvements were observed, echocardiographic systolic function improved after immunosuppressive therapy administration. On the 10th day of ECMO assistance, cardiac transplantation seemed to be the only solution; however, the family members did not consent. After 2 weeks of ECMO support, upper gastrointestinal bleeding combined with hemolysis prevented the continuation of mechanical support. Therefore, high-dose inotropic support (20 ╬╝g/min norepinephrine and 20 ╬╝g/kg/min dobutamine) was administered and the ECMO and temporary pace-maker were discontinued. All intravenous medications were discontinued 1 month after ECMO cessation due to poor patient condition. An echocardiogram showed that the LVEF was 21%, and the junctional escape rhythm with an intraventricular conduction disturbance was still noted (QRS duration, 144 msec; Fig. 1C). The patientŌĆÖs activities were limited due to dyspnea (50%, Karnofsky Performance Status Scale [3]); however, he was discharged 2 months after ECMO cessation on low doses of angiotensin converting enzyme inhibitors and beta blockers. Four months after ECMO cessation, echocardiography and ECG were performed. Severe global systolic dysfunction (LVEF, 25%) was observed on echocardiography. However, a narrow QRS rhythm was found (QRS duration, 104 msec; Fig. 2B). Significant systolic function improvement (LVEF, 46%, Fig. 1D and 2C) was observed 6 months after discharge and the patientŌĆÖs Karnofsky Performance Status Scale score had increased to 90%.
DISCUSSION
Fulminant myocarditis is life-threatening in its acute phase due to markedly decreased cardiac function. However, recovery is often complete after the early decompensated phase, and if the patient survives the acute active phase without complications, the long-term prognosis is excellent [2]. Recently, a venoarterial ECMO device, which is widely used for cardiogenic shock and cardiac arrest, has been introduced for the treatment of fulminant myocarditis. It is a more accessible and involves a reversible approach compared with left ventricular assist devices [4]. In a recent meta-analysis, more than two-thirds of the patients presenting with shock or arrest survived with ECMO support [5]. While immunosuppression has no known effective role in acute myocarditis, potent hemodynamic support is a standard treatment for acute fulminant myocarditis with hemodynamic compromise [6]. Most patients with acute active myocarditis have early, spontaneously improved cardiac function post-presentation; typically, hemodynamic support with ECMO is required for approximately 1 week, unlike our case. The median duration of ECMO support in most cases is 5 to 7 days [7].
Our patient eventually showed delayed improvement of cardiac function after removing the ECMO support. The possibility of delayed improvement makes the decision regarding cardiac transplantation during the early period of ECMO support a challenge, as postponing cardiac transplantation may lead to patient mortality. There are no definitive guidelines for cardiac transplantation during the acute period of fulminant myocarditis; very low LVEF itself is not an indication for cardiac transplantation. As in this case, complications may occur with prolonged ECMO support, and the recovery period from the acute phase of fulminant myocarditis is indeterminate. If no recovery occurs within 1 week of ECMO support, there are two management options: 1) continue with ECMO support and improvements may occur and 2) intervene with long-term ventricular device implantation or cardiac transplantation. If the patient is hemodynamically stable after ECMO removal, the transplantation decision should be based on current candidate selection guidelines for cardiac transplantation.
Various ECG changes, including ST-segment and T-wave abnormalities, have been reported in fulminant myocarditis, with serious conduction disturbances associated with more severe histological myocardial necrosis and poorer prognoses [8]. Abnormal QRS complexes have more severe clinical courses and a higher mortality rate. A recent study reported that prolonged QRS duration is an independent predictor of cardiac death and the need for heart transplantation [9]. However, the ECG changes were evaluated at specific time points during hospitalization, and ECG changes in fulminant myocarditis could present any time after admission [9]. In this case, the QRS was prolonged before recovery, but subsequently narrowed with improvements in cardiac function. Therefore, ECG changes are a possible indicator of myocardial disease status. Generally, ECG abnormalities improve after the acute phase. Lee et al. [10] reported the reversal of various ECG changes in a patient within 7 days. A wide QRS was also observed in our case; however, contrary to Lee et al. [10], ECG changes, including the presence of a P wave and shortening of the QRS duration, were observed much later (6 months after stopping ECMO support, Fig. 2C). The treatment for arrhythmic complications during myocarditis is predominantly supportive; if arrhythmias persist after the recovery of left ventricular function, they are treated accordingly.
In conclusion, we cautiously suggest that close observation is possible for up to half a year before considering cardiac transplantation for fulminant myocarditis patients who are hemodynamically stable after ECMO cessation.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print






